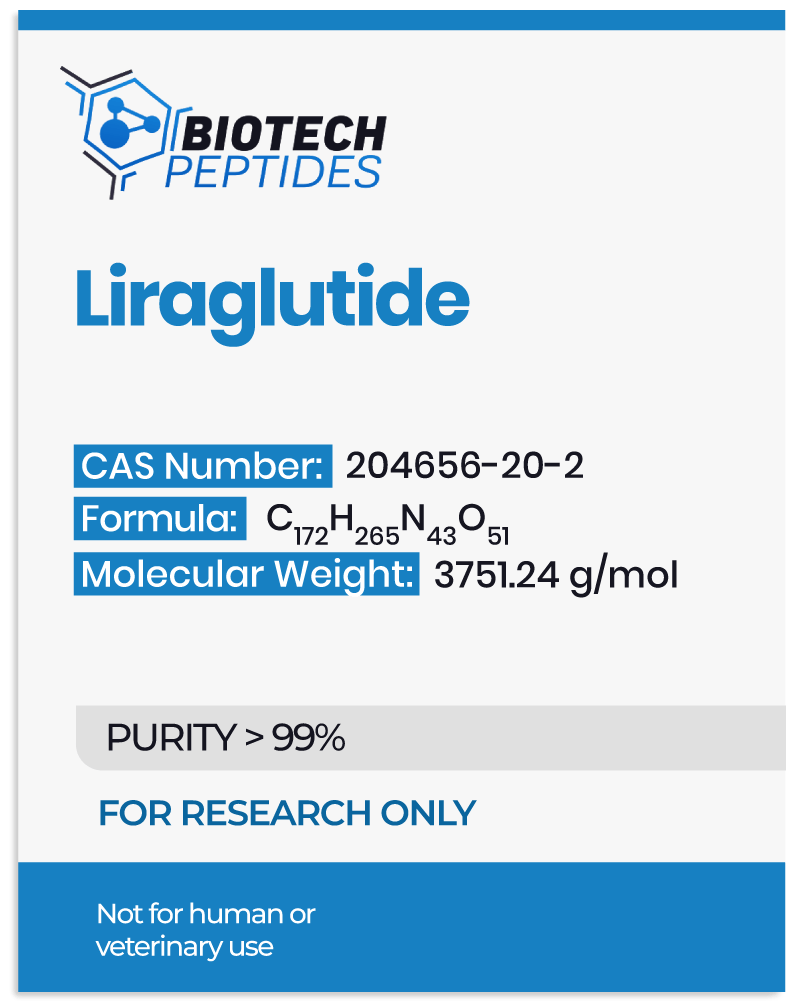
Liraglutide peptide was first developed in the 1990s, with the intention to improve blood sugar control in cases of type 2 diabetes. The development of Liraglutide was based on the discovery of the hormone glucagon-like peptide-1 (GLP-1), which is involved in regulating blood sugar levels. It has been widely researched since its development, with some studies outlined below.
Liraglutide Peptide and Body Composition
Liraglutide peptide may have significant potential in regulation of weight and lean mass. One study focused on obese and overweight research models, with findings exhibiting a loss of at least 5% of initial weight.[1] The models were randomly exposed either to Liraglutide peptide or a placebo for 56 weeks, which appeared to lead to additional weight loss on average for the study period. Liraglutide peptide also appeared to produce small improvements in some cardiovascular risk factors. The scientists reported that “From randomization to week 56, weight decreased an additional mean 6.2% […] with liraglutide and 0.2% […] with placebo.” Another 56-week-long study reported similar results, with an average of 5-6% of observed weight loss in most models under observation.[2]
Researchers also suggested that Liraglutide peptide and prolonged physical activity may lead to a 2-fold rate of weight loss compared controls subjected to physical activity alone.[3] One of the longest studies to investigate the action of Liraglutide peptide in weight was a 20-week randomized, double-blind, placebo-controlled study with a 2-year extension involving 564 overweight research models.[4] Receiving either Liraglutide peptide, a placebo, or an open-label weight loss compound in addition to carefully monitored nutritional intake and physical output. The study’s results suggested that Liraglutide peptide-exposed models lost more weight than those on a placebo or compound. Moreover, the research models exposed to Liraglutide also appeared to experience improvements in metabolic syndrome and blood pressure.
Liraglutide and the Endocrine System
Liraglutide peptide has some potential to act as an incretin in the endocrine system, specifically in the pancreas, which enhances insulin secretion. The peptide was developed to mimic the action of the incretin hormone GLP-1, which stimulates insulin secretion and reduces glucagon secretion in a glucose-dependent manner.[5] The effect appears to depend on the serum glucose levels, diminishing if the glucose is low and thereby preventing the occurrence of hypoglycemia.
When Liraglutide peptide was delivered to research models of type 2 diabetes, studies suggest it may enhance the incretin effect by increasing GLP-1 levels in the bloodstream. This leads to increased insulin secretion and decreased glucagon secretion, resulting in improved blood sugar control. These studies reported apparently significant improvements in various parameters related to blood sugar control in model of type 2 diabetes.[6] These included improved levels of glycated hemoglobin, body mass index (BMI), cardiovascular parameters, etc., all within the 3-6 months of the study. The scientists reported that the “meaningful difference in weight, body mass index, glycated hemoglobin (HbA1C), systolic blood pressure, and diastolic blood pressure from baseline to follow-up was -5.36 kg, -2.14 kg/m2, -1.76%, -12.38 mmHg, and 5.55 mmHg, respectively.” In addition, experiments observed that Liraglutide might also exhibit a protective action on the function of the pancreas in type 2 diabetes cases and preserve the function of the beta cells, which normally produce insulin.[7]
Liraglutide Peptide and the Digestive System
Liraglutide peptide may slow down the emptying of food from the stomach into the small intestine, leading to prolonged satiety and reduced appetite. Research studies suggest that 1-h gastric emptying was, on average, 23% lower in studies than controls, although that performance appeared to be concentration-dependent.[8] Scientists reported that the speed of gastric emptying eventually returned to normal after 4 hours.
Liraglutide Peptide and the Nervous System
Apart from slowing down gastric emptying, Liraglutide peptide has also been suggested by researchers to suppress appetite by directly affecting the brain via reduced hunger hormone signaling. This potential may be due to the peptide’s hypothetical interaction with GLP-1 receptors in the brain, whose activation may lead to reduced appetite.[9] Liraglutide peptide has suggested promise in neuroprotection, as reported in murine models of Parkinson’s Disease (PD),[10] with scientists suggesting that the peptide might reduce neuroinflammation and reduce neuron loss.
PD is a neurodegenerative disorder that affects the nervous system, particularly the dopaminergic neurons in the brain. While the exact cause of PD is unknown, some data suggests that an autoimmune reaction that destroys these neurons may contribute to the development of the disease.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study [published correction appears in Int J Obes (Lond). 2013 Nov;37(11):1514] [published correction appears in Int J Obes (Lond). 2015 Jan;39(1):187]. Int J Obes (Lond). 2013;37(11):1443-1451. doi:10.1038/ijo.2013.120
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of Liraglutide for Weight Loss Among Patients With Type 2 Diabetes: The SCALE Diabetes Randomized Clinical Trial [published correction appears in JAMA. 2016 Jan 5;315(1):90]. JAMA. 2015;314(7):687-699. doi:10.1001/jama.2015.9676
- Lundgren JR, Janus C, Jensen SBK, et al. Healthy Weight Loss Maintenance with Exercise, Liraglutide, or Both Combined. N Engl J Med. 2021;384(18):1719-1730. doi:10.1056/NEJMoa2028198
- Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide [published correction appears in Int J Obes (Lond). 2012 Jun;36(6):890] [published correction appears in Int J Obes (Lond). 2013 Feb;37(2):322]. Int J Obes (Lond). 2012;36(6):843-854. doi:10.1038/ijo.2011.158
- Neumiller JJ, Campbell RK. Liraglutide: a once-daily incretin mimetic for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2009;43(9):1433-1444. doi:10.1345/aph.1M134
- Zameer R, Kamin M, Raja U, et al. Effectiveness, Safety, and Patient Satisfaction of Liraglutide in Type 2 Diabetic Patients. Cureus. 2020;12(8):e9937. Published 2020 Aug 22. doi:10.7759/cureus.9937
- Kapodistria K, Tsilibary EP, Kotsopoulou E, Moustardas P, Kitsiou P. Liraglutide, a human glucagon-like peptide-1 analogue, stimulates AKT-dependent survival signalling and inhibits pancreatic β-cell apoptosis. J Cell Mol Med. 2018;22(6):2970-2980. doi:10.1111/jcmm.13259
- van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond). 2014;38(6):784-793. doi:10.1038/ijo.2013.162
- Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15(3):181-187. doi:10.1007/s11154-014-9289-5
- Cao B, Zhang Y, Chen J, Wu P, Dong Y, Wang Y. Neuroprotective effects of liraglutide against inflammation through the AMPK/NF-κB pathway in a mouse model of Parkinson’s disease. Metab Brain Dis. 2022;37(2):451-462. doi:10.1007/s11011-021-00879-1