Contents:
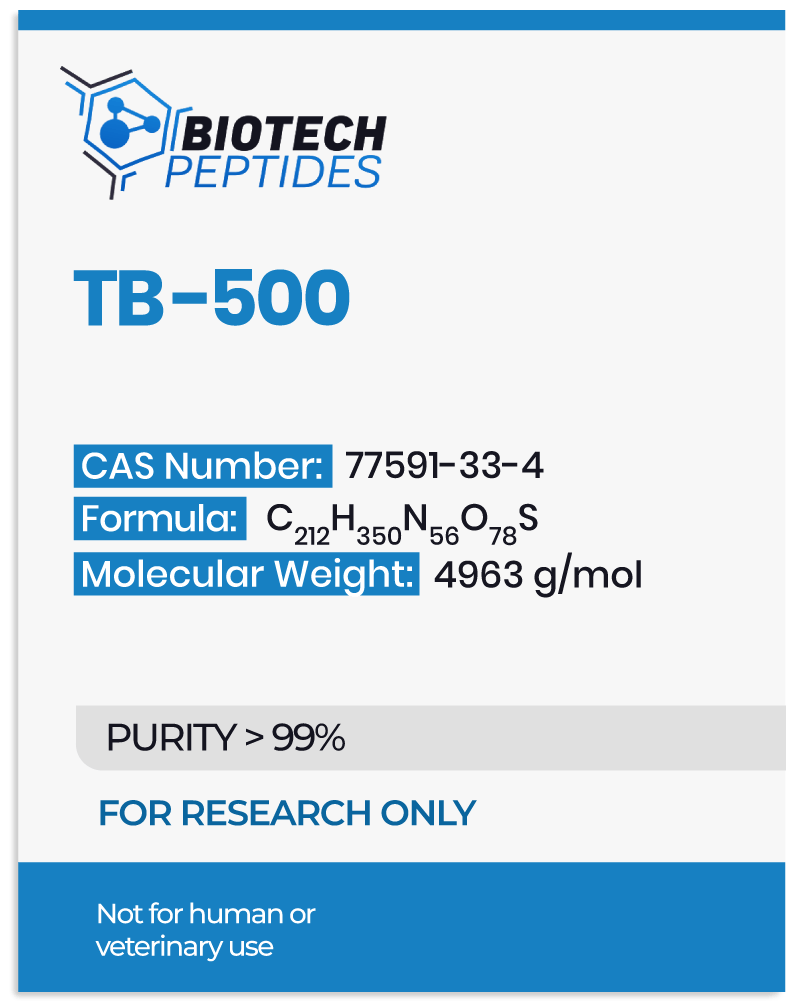
According to scientific data, TB-500 is a synthetic peptide with wound healing and anti-inflammatory potential.[2] This peptide differs from others in that it appears to promote keratinocyte and endothelial migration. It has a low molecular weight and does not appear to bind to the extracellular matrix, implying that it may potentially travel long distances through tissues. The most important mechanism of action of the TB-500 peptide is its potential to modulate actin activity.
TB-500 Peptide Research
TB-500 peptide may be concentrated at injury sites, where it may improve wound healing and repair in the brain, spinal cord, skin, heart, bones, and organs.[4]
When released from platelets, TB-500 peptide may play a potential cellular role in immune regulation and inflammation. As a result, TB-500 peptide may increase B cells, which regulate antibody activation. It may increase Actin levels to promote tissue repair after injury and potentially stimulate T cell synthesis to improve immune system function.[5]
TB-500 and Blood Clots: TB-500 peptide may be a vital ancillary in mitigating blood clots and might regulate the formation of blood vessels.
TB-500 and Soft Tissue Damage: The potential of TB-500 peptide to promote angiogenesis and reduce inflammation may result in muscle, ligament, and tendon recovery.
TB-500 and Muscular Function: TB-500 peptide may potentially increase the rate of muscle repair and growth rate, including regulating muscle spasms.
TB-500 and Neurological and Cardiovascular Damage: TB-500 peptide may potentially promote angiogenesis, including neuron formation and better brain axonal density.
TB-500 and Matrix Metalloproteinase Expression in Tissue Repair: Wound healing impairment is common in diabetic cases of immobility. According to research, TB-500 peptide may potentially improve dermal wound repair in rats, dB/dB diabetic mice, and aged mice.[6] Philip et al. concluded “that thymosin β4 is active for wound repair in models of impaired healing and may have efficacy in chronic wounds.” In normal rats and mice, the peptide appears to potentially promote corneal repair. TB-500 may regulate matrix metalloproteinase (MMP) expression in wound repair cells. RT-PCR analysis of whole excised mouse dermal wounds on days 1, 2, and 3 after injury suggested that TB-500 peptide increased the expression of several metalloproteinases, including MMP-2 and -9, by several folds on days two after wounding. The metalloproteinases secreted by activated monocytes in response to exogenous TB-500 in the wound were also studied. They suggested that the peptide increased MMP-1 and MMP-9 levels.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Ho, E. N., Kwok, W. H., Lau, M. Y., Wong, A. S., Wan, T. S., Lam, K. K., Schiff, P. J., & Stewart, B. D. (2012). Doping control analysis of TB-500 peptide, a synthetic version of an active region of thymosin β₄, in equine urine and plasma by liquid chromatography-mass spectrometry. Journal of chromatography. A, 1265, 57–69. https://doi.org/10.1016/j.chroma.2012.09.043
- Grant, D. S., Rose, W., Yaen, C., Goldstein, A., Martinez, J., & Kleinman, H. (1999). Thymosin beta4 enhances endothelial cell differentiation and angiogenesis. Angiogenesis, 3(2), 125–135. https://doi.org/10.1023/a:1009041911493
- Malinda, K. M., Sidhu, G. S., Mani, H., Banaudha, K., Maheshwari, R. K., Goldstein, A. L., & Kleinman, H. K. (1999). Thymosin beta4 accelerates wound healing. The Journal of investigative dermatology, 113(3), 364–368. https://doi.org/10.1046/j.1523-1747.1999.00708.x
- Goldstein, A. L., Hannappel, E., & Kleinman, H. K. (2005). Thymosin β4: actin-sequestering protein moonlights to repair injured tissues. Trends in molecular medicine, 11(9), 421-429.
- Huff, T., Otto, A. M., Müller, C. S., Meier, M., & Hannappel, E. (2002). Thymosin β4 is released from human blood platelets and attached by factor XIIIa (transglutaminase) to fibrin and collagen. The FASEB journal, 16(7), 691-696.
- Philp, D., Badamchian, M., Scheremeta, B., Nguyen, M., Goldstein, A. L., & Kleinman, H. K. (2003). Thymosin β4 and a synthetic peptide containing its actin‐binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound repair and regeneration, 11(1), 19-24.