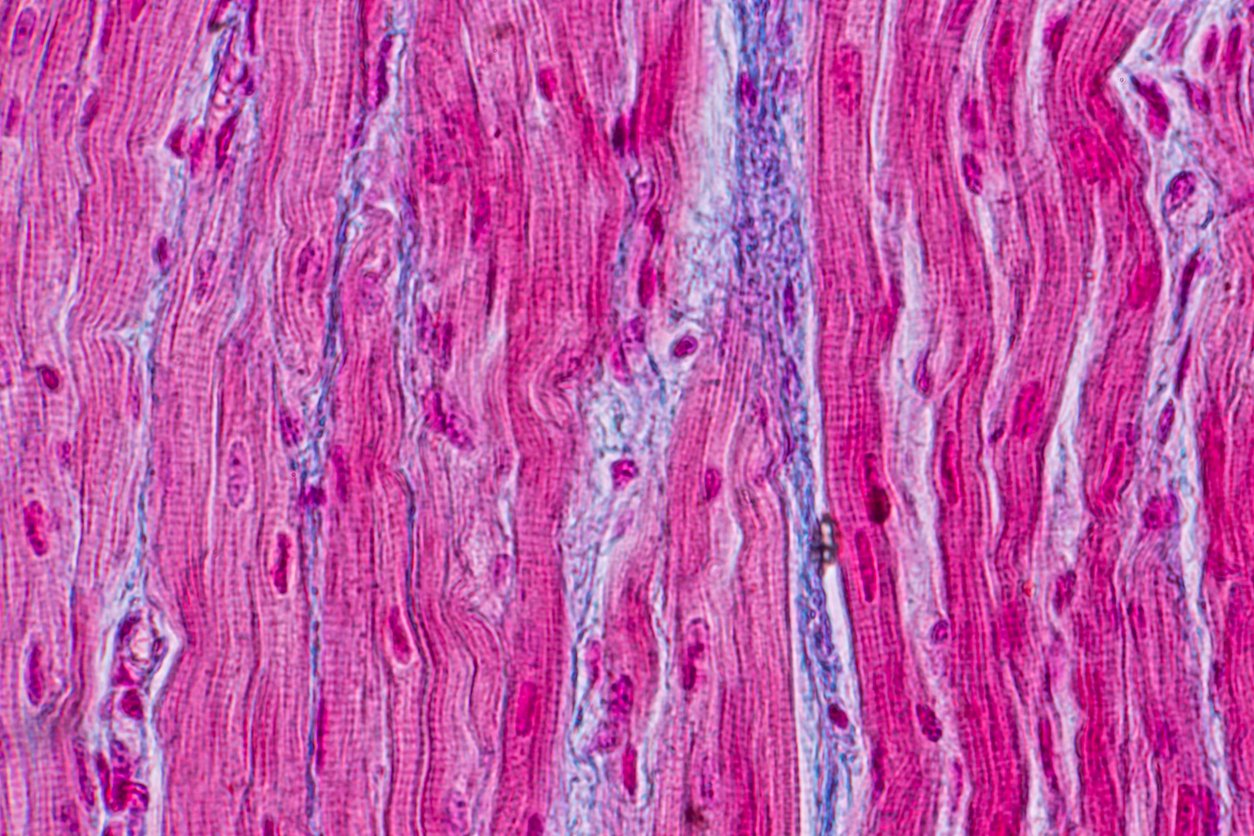
Research studies suggest that Thymosin Beta 4 may exhibit some potential to modulate actin dynamics, a process that is potentially responsible for cell movement and maintaining structural integrity. This property seems to be particularly relevant in cellular processes such as cell migration and tissue repair. Moreover, Thymosin Beta 4 is hypothesized to inhibit inflammatory cytokines and possibly reduce oxidative stress, which may contribute to its protective potential at a cellular level of cardiac cells.[1]
Research also posits that Thymosin Beta 4 might stimulate stem cell differentiation and possibly angiogenesis (the formation of new blood vessels), which are thought to be crucial processes in the life cycle of cells including cardiac cells. More specifically, it’s been proposed that Thymosin Beta 4 might interact with cardiac progenitor cells, and stimulate their migration. Hypothetically, this peptide might also affect processes such as cardiac cell proliferation or survival, which are also key aspects of potentially facilitating faster cardiac regeneration.[2]
Thymosin Beta 4 Peptide Potential Mechanism of Action
The mechanisms of action for Thymosin Beta 4 are still being explored. Nevertheless, there are some preliminary insights into its potential activity. For example, it seems to function as an actin-binding protein that may inhibit the polymerization of globular actin (G-actin) into filamentous actin (F-actin). This process is known as actin sequestration, which could potentially cause in elevated G-actin levels.[3] [4] [5] Actin, which appears to be a major component of the cellular cytoskeleton including cardiac cells, may provide structural support to cells and is involved in various cellular processes, including cardiac cell motility to facilitate regeneration. Thymosin Beta 4 is thought to bind with actin primarily (but not exclusively) via its central actin-binding domain (aa 17-23), also known as Ac-LKKTETQ.[6] Researchers posit that “thymosin beta(4) has the potential for significant roles in tissue development, maintenance, repair, and pathology” including cardiac regeneration. The potential prevention of F-actin polymerization by Thymosin Beta 4 might alter the cellular cytoskeleton, which could affect the ability of cardiac cells to move and change shape. This process could have implications in various physiological and pathological processes where cardiac cell motility is crucial, such as cardiac regeneration.[7] It’s important to note that these mechanisms are hypothesized to explain the potential action of intracellular Thymosin Beta 4.
Additionally, TB4 may also hold potential when present outside of cardiac cells (extracellularly).[8] Some research in blood vessel cells suggests that the influence of extracellular Thymosin Beta 4 might regulate processes such as cardiac cell motility and angiogenesis. Scientists posit that the peptide might regulate these processes by interacting with cell surface-located ATP synthase enzymes. These are cellular enzymes considered to be involved in the energy production of the cardiac and other cells.[9] Extracellular Thymosin Beta 4 might also potentially become oxidized in sites of inflammation to Thymosin Beta 4 sulfoxide, and the latter is thought to have potent anti-inflammatory properties.[10] Furthermore, Thymosin Beta 4 might also reduce inflammation by possibly increasing the expression of microRNA-146a (miR-146a). This could potentially decrease the expression of two pro-inflammatory cytokines, called L-1 receptor-associated kinase 1 (IRAK1) and tumor necrosis factor receptor-associated factor 6 (TRAF6).[11] Apparently reducing inflammation and potentially stimulating cardiac cell motility is thought to play a major role in tissue regeneration, including cardiac recovery.
Thymosin Beta 4 Peptide and Regeneration of Cardiac Cells
Experimental studies suggest that endothelial progenitor cells (EPC) may have a potential for inducing cardiac regeneration, and the addition of TB4 appears to enhance this process. The researchers posited that EPC with addition of Thymosin Beta 4 peptide may have approved the apparent cardiac function in damaged myocardium and facilitated cardiac repair.[12] This was likely due to its potential action on cardiac cell motility and apparent stimulation of cardiac cell progenitors. Thymosin Beta 4 may also have potentially contributed to cardiac regeneration by apparently reducing inflammation. The peptide has been suggested to potentially decrease reactive oxygen species (ROS) and lipid peroxidation while possibly increasing antioxidant levels. It may also inhibit the activation of nuclear factor kappa B, thus apparently suppressing pro-inflammatory cytokine production, and preventing fibrosis – a potentially undesired regeneration process known to impede tissue function.[13] These findings suggest that Thymosin β4 and cardiac reprogramming technology might work synergistically to limit damage to the heart and promote cardiac regeneration, possibly also through the stimulation of endogenous cells within the heart. Thymosin Beta 4 might also promote myocardial survival in hypoxic conditions and apparently stimulates angiogenesis, which could potentially lead to cardiac repair. The researchers also propose a potential mechanism involving the reprogramming of cardiac fibroblasts to cardiomyocyte-like cells[14] Ultimately, the authors commented that “thymosin β4 and cardiac reprogramming technology may synergistically limit damage to the heart and promote cardiac regeneration through the stimulation of endogenous cells within the heart.” A study in murine models of coronary artery ligation also reported that Thymosin Beta 4 application may upregulate integrin-linked kinase (ILK) and protein kinase B activity in the heart, potentially enhancing early myocyte survival and apparently improving cardiac function.[15] The scientists also posited that “Thymosin Beta 4 peptide promotes myocardial and endothelial cell migration in the embryonic heart and retains this property in postnatal cardiomyocytes.”
Conclusion
In conclusion, Thymosin Beta 4 appears to have a complex and multifaceted role in cellular processes related to regeneration. That role appears to be particularly in relation to Thymosin Beta 4’s apparent potential on actin dynamics and cell motility including cardiac cells. Its potential influence on cardiac cells and its possible involvement in processes such as inflammation reduction and angiogenesis also suggest that it could play a significant role in cardiac regeneration. Most importantly, Thymosin Beta 4 may also upregulate cardiac cell progenitors, and via its apparent effects on cell motility, it may aid their relocation to injury sites for recovery and regeneration.
However, these are preliminary findings and the exact mechanisms of Thymosin Beta 4’s action, both intracellularly and extracellularly, remain to be fully elucidated. The apparent action of TB4 in the context of cardiac cell regeneration is particularly intriguing, but further research is needed to substantiate these claims and to explore the full range of Thymosin Beta 4’s potential.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Goldstein, A. L., Hannappel, E., Sosne, G., & Kleinman, H. K. (2012). Thymosin β4: a multi-functional regenerative peptide. Basic properties and clinical applications. Expert opinion on biological therapy, 12(1), 37–51. https://doi.org/10.1517/14712598.2012.634793
- Choudry, F. A., Yeo, C., Mozid, A., Martin, J. F., & Mathur, A. (2015). Increases in plasma Tβ4 after intracardiac cell therapy in chronic ischemic heart failure is associated with symptomatic improvement. Regenerative medicine, 10(4), 403–410. https://doi.org/10.2217/rme.15.9
- Sanders, M. C., Goldstein, A. L., & Wang, Y. L. (1992). Thymosin Beta 4 (Fx peptide) is a potent regulator of actin polymerization in living cells. Proceedings of the National Academy of Sciences of the United States of America, 89(10), 4678–4682. https://doi.org/10.1073/pnas.89.10.4678
- Irobi, E., Aguda, A. H., Larsson, M., Guerin, C., Yin, H. L., Burtnick, L. D., Blanchoin, L., & Robinson, R. C. (2004). Structural basis of actin sequestration by thymosin-beta4: implications for WH2 proteins. The EMBO journal, 23(18), 3599–3608. https://doi.org/10.1038/sj.emboj.7600372
- Belsky, J. B., Rivers, E. P., Filbin, M. R., Lee, P. J., & Morris, D. C. (2018). Thymosin Beta 4 regulation of actin in sepsis. Expert opinion on biological therapy, 18(sup1), 193-197.
- Sosne, G., Qiu, P., Goldstein, A. L., & Wheater, M. (2010). Biological activities of thymosin beta4 defined by active sites in short peptide sequences. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 24(7), 2144–2151. https://doi.org/10.1096/fj.09-142307
- Yadav, T., Gau, D., & Roy, P. (2022). Mitochondria-actin cytoskeleton crosstalk in cell migration. Journal of cellular physiology, 237(5), 2387–2403. https://doi.org/10.1002/jcp.30729
- Huff, T., Müller, C. S., Otto, A. M., Netzker, R., & Hannappel, E. (2001). beta-Thymosins, small acidic peptides with multiple functions. The international journal of biochemistry & cell biology, 33(3), 205–220. https://doi.org/10.1016/s1357-2725(00)00087-x
- Freeman, K. W., Bowman, B. R., & Zetter, B. R. (2011). Regenerative protein thymosin beta-4 is a novel regulator of purinergic signaling. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 25(3), 907–915. https://doi.org/10.1096/fj.10-169417
- Young, J. D., Lawrence, A. J., MacLean, A. G., Leung, B. P., McInnes, I. B., Canas, B., Pappin, D. J., & Stevenson, R. D. (1999). Thymosin Beta 4 sulfoxide is an anti-inflammatory agent generated by monocytes in the presence of glucocorticoids. Nature medicine, 5(12), 1424–1427. https://doi.org/10.1038/71002
- Santra, M., Zhang, Z. G., Yang, J., Santra, S., Santra, S., Chopp, M., & Morris, D. C. (2014). Thymosin β4 up-regulation of microRNA-146a promotes oligodendrocyte differentiation and suppression of the Toll-like proinflammatory pathway. The Journal of biological chemistry, 289(28), 19508–19518. https://doi.org/10.1074/jbc.M113.529966
- Zhu, J., Song, J., Yu, L., Zheng, H., Zhou, B., Weng, S., & Fu, G. (2016). Safety and efficacy of autologous thymosin β4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: A pilot study. Cytotherapy, 18(8), 1037–1042. https://doi.org/10.1016/j.jcyt.2016.05.006
- Shah, R., Reyes-Gordillo, K., Cheng, Y., Varatharajalu, R., Ibrahim, J., & Lakshman, M. R. (2018). Thymosin β4 Prevents Oxidative Stress, Inflammation, and Fibrosis in Ethanol- and LPS-Induced Liver Injury in Mice. Oxidative medicine and cellular longevity, 2018, 9630175. https://doi.org/10.1155/2018/9630175
- Srivastava, D., Ieda, M., Fu, J., & Qian, L. (2012). Cardiac repair with thymosin β4 and cardiac reprogramming factors. Annals of the New York Academy of Sciences, 1270, 66–72. https://doi.org/10.1111/j.1749-6632.2012.06696.x
- Bock-Marquette, I., Saxena, A., White, M. D., Dimaio, J. M., & Srivastava, D. (2004). Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature, 432(7016), 466–472. https://doi.org/10.1038/nature03000