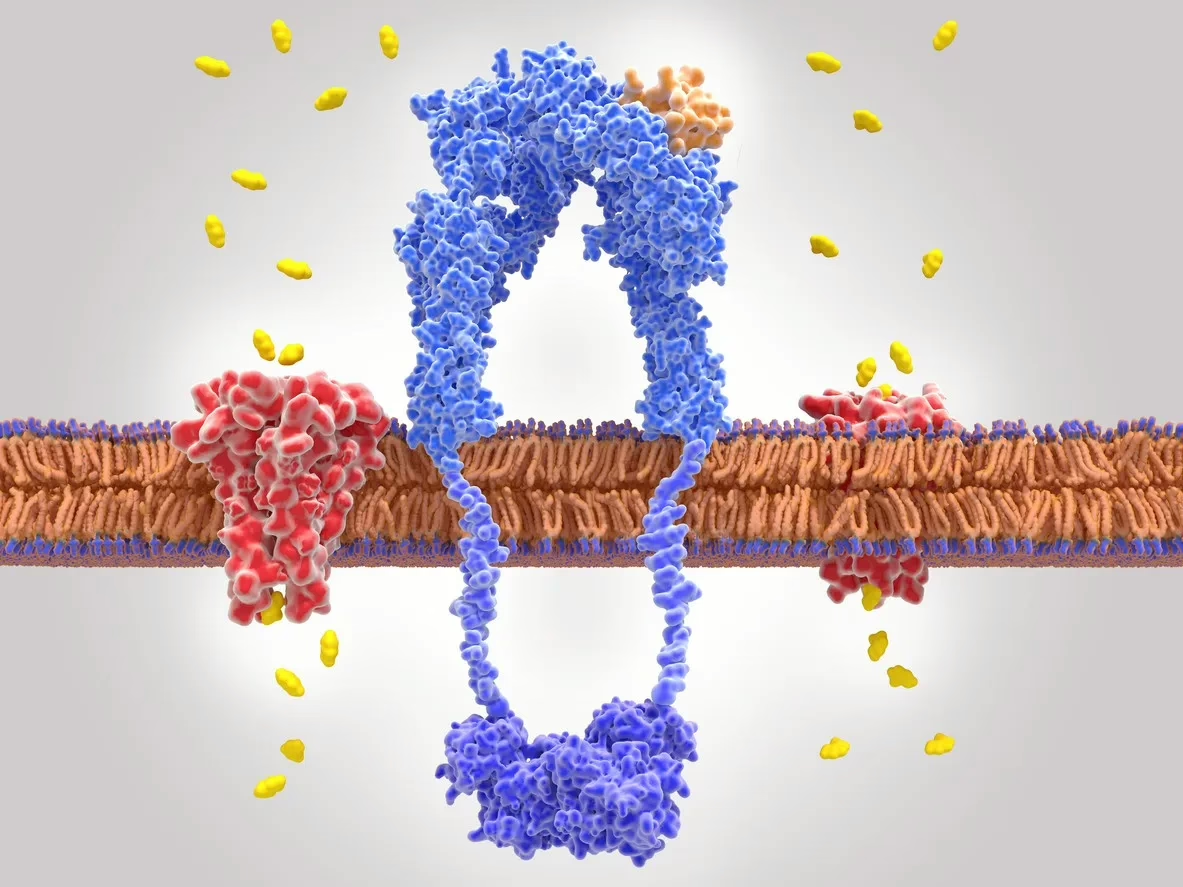
Designed as an analog to gastric inhibitory polypeptide (GIP), this compound reportedly exhibits additional affinity towards the glucagon-like peptide-1 receptor and the glucagon (GCG) receptor.[2] These receptors typically bind to their respective endogenous hormones, GIP, GLP-1, and GCG, which function as key hormonal regulators within the endocrine system. It is proposed that GLP-1 and GIP, acting as incretin hormones, might enhance insulin secretion from pancreatic beta cells and potentially increase satiety following nutrient intake. Conversely, glucagon is thought to play a compensatory role by potentially elevating blood glucose levels during fasting states.
Moreover, activation of GLP-1 receptors is suggested to slow gastric emptying, while stimulation of GCG receptors is hypothesized to increase energy expenditure and fat metabolism, notably influencing hepatic processes and converting white adipose tissue to beige adipose tissue, which is believed to possess thermogenic properties similar to brown adipose tissue, thereby potentially enhancing thermogenesis and metabolic rates.
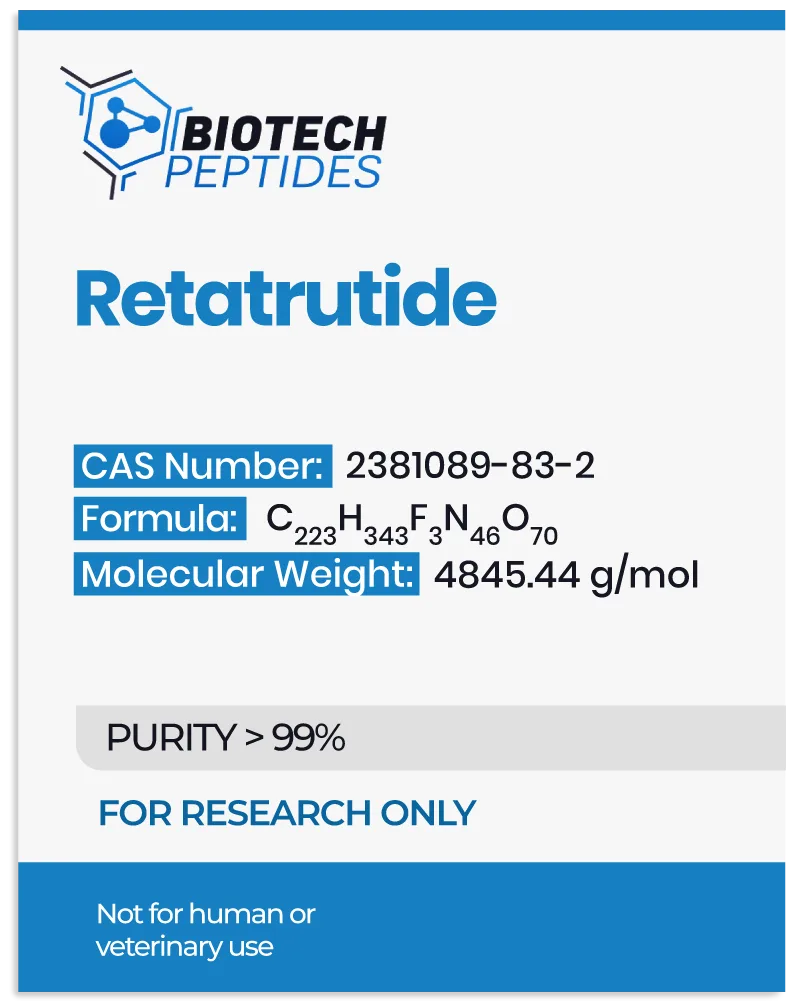
The interaction of Retatrutide with these receptors indicates a multifaceted impact on metabolic regulation, which may be significant in the study of glycemic control and weight management. Additionally, Retatrutide has undergone chemical modification with a C20 moiety, purportedly extending its half-life to approximately six days.[3]
Scientific and Research Studies
Retatrutide Peptide and Glycated Hemoglobin Levels
Retatrutide has indicated potential in reducing glycated hemoglobin (HbA1c) levels in models of hyperglycemia and baseline HbA1c exceeding 7%. Data from phase 2 trials lasting up to 36 weeks suggest that Retatrutide may decrease HbA1c levels by as much as 2.16% (23.59 mmol/mol), indicating a significant amelioration of hyperglycemia. The findings also highlighted a notable reduction in total weight, with decreases from baseline to 36 weeks reaching up to a least-squares mean of 16.94% in the Retatrutide-exposed groups.[4]
Retatrutide Peptide and Appetite and Energy Intake
In a 48-week phase 2 study, Retatrutide has been investigated for its potential to significantly reduce total energy intake and facilitate the maintenance of a caloric deficit.
The research suggests that Retatrutide may lead to a substantial reduction in baseline weight, with observed decreases exceeding 24.2%, compared to a 2.1% reduction observed in the control group. Additionally, the study reported improvements in several cardiometabolic parameters, including indicators related to cholesterol metabolism, glucose regulation, and insulin sensitivity.[5]
Researchers noted that in type 2 diabetes models, Retatrutide peptide showed “meaningful improvements in glycaemic control and robust reductions in bodyweight, with a […] profile consistent with GLP-1 receptor agonists and GIP and GLP-1 receptor agonists.”
Retatrutide Peptide and GIP Receptors Interaction
Retatrutide’s mechanism of action includes the activation of gastric inhibitory polypeptide (GIP) receptors. A comprehensive study has investigated the possible intricate roles of GIP receptor activation in the regulation of energy balance. Researchers hypothesize that Retatrutide may exert influence through central neural pathways, particularly in key brain regions such as the hypothalamus and brainstem[6], which are considered critical for maintaining energy homeostasis and appetite. It is proposed that GIP receptor agonists may directly interact with neurons in these regions, potentially altering neuronal activity to reduce caloric intake and promote a negative energy balance.
The hypothalamus, a regulator in this process, contains specific nuclei—such as the arcuate, paraventricular, and ventromedial nuclei—that are believed to be essential in the regulation of appetite and energy expenditure. Activation of GIP receptors within these nuclei may suppress appetite or enhance signals of satiety. For example, GIP receptor activation in the arcuate nucleus might modulate neurons producing neuropeptide Y (NPY), which is associated with increased appetite, and pro-opiomelanocortin (POMC), which is linked to appetite suppression. This suggests that GIP receptor agonists might influence these neuropeptide systems, potentially leading to reduced caloric intake.
Moreover, the study explores the potential impact of GIP receptor agonists on the brain’s emetic centers. By potentially modulating neural circuits in the area postrema and the nucleus tractus solitarius of the brainstem—key areas involved in nausea and vomiting—GIP receptor agonists might inhibit these responses. Additionally, the role of GIP receptor agonists in enhancing the permeability of the blood-brain barrier (BBB) is proposed as a mechanism to improve the delivery and efficacy of agents targeting brain regions involved in energy balance. This may involve the modulation of tight junctions or transport systems within the neurovascular unit, facilitating increased access of these agents to the central nervous system (CNS).
Retatrutide Peptide and Energy Metabolism
Activation of glucagon (GCG) receptors by agonists such as Retatrutide may promote increased energy expenditure and fat oxidation, potentially enhancing energy utilization. Studies suggest that Retatrutide may activate GCG receptors in hepatocytes, possibly boosting energy expenditure by increasing lipid catabolism and enhancing the liver’s metabolic rate. This might be facilitated through mechanisms such as hepatic futile cycling, enhanced mitochondrial function, and the secretion of thermogenic agents such as fibroblast growth factor 21 (FGF21) and bile acids, which may further augment energy expenditure. The activation of GCG receptors in the liver by Retatrutide may trigger a cascade of metabolic events that induce weight loss, including the reduction of hepatic steatosis through increased lipid oxidation, elevated activity of metabolic enzymes, and upregulation of mitochondrial biogenesis.[7] Key hormones like FGF21 and bile acids, released by the liver in response to GCG receptor activation, might play a role in systemic energy homeostasis.
Retatrutide may also stimulate GCG receptors in adipocytes, initiating a process known as beiging, which transforms white adipocytes into beige adipocytes. Beige adipocytes, similar to brown adipocytes, may contribute to increased caloric expenditure by generating heat, potentially enhancing thermogenesis and metabolic rate. The beiging of white adipose tissue may involve the induction of UCP1-dependent non-shivering thermogenesis and metabolic futile cycles, such as the creatine and succinate cycles, which may dissipate energy as heat. Additionally, Retatrutide might upregulate the expression of thermogenic genes in both brown and beige adipocytes, promoting the formation and activity of these thermogenically active cells.
Furthermore, Retatrutide’s activation of GCG receptors may extend to enhancing the thermogenic capacity of brown adipose tissue (BAT). Activation of BAT by Retatrutide may increase metabolic rate through uncoupling protein 1 (UCP1), which may dissipate the mitochondrial proton gradient as heat. This process might utilize stored lipids and support the oxidation of circulating glucose, lipids, and amino acids, contributing to overall energy expenditure. Based on this research, the researchers propose that “the thermogenic activity of GCGR agonism” may assist “in lowering weight.”
Retatrutide Peptide and Metabolic Influence
Research posits that Retatrutide may exert its effects through the activation of glucagon-like peptide-1 (GLP-1) receptors, which are typically ubiquitous. This peptide potentially interacts with GLP-1 receptors in the pancreas, which may stimulate insulin secretion from pancreatic beta cells and reduce glucagon production from alpha cells in a glucose-dependent manner. The GLP-1 receptor, a member of the class B G protein-coupled receptor family, is thought to primarily engage the cAMP-PKA signaling pathway in the pancreas. The interaction between GLP-1 and its receptor is believed to activate adenylate cyclase (AC), which converts ATP to cyclic adenosine monophosphate (cAMP), potentially increasing cAMP levels. This elevation in cAMP might activate protein kinase A (PKA) and the guanine nucleotide exchange factor RAPGEF4 (also known as EPAC2). The activated PKA may close ATP-sensitive K+ channels and depolarize the cell membrane, possibly activating voltage-dependent Ca2+ channels, leading to Ca2+ influx and the generation of action potentials. Furthermore, PKA might facilitate the release of Ca2+ by activating inositol trisphosphate (IP3). The activated EPAC2 may also activate Ras-related protein 1 and phospholipase C, possibly engaging the IP3 and diacylglycerol (DAG) pathways to further enhance Ca2+ release. Collectively, these pathways may increase intracellular Ca2+ levels, potentially boosting mitochondrial ATP synthesis and promoting insulin secretion via exocytosis.[7]
Similar to its effects on GIP receptors, Retatrutide is suggested to influence neurons in the hypothalamic arcuate nucleus, involved in the regulation of appetite and hunger, possibly through GLP-1 receptors. These neurons express neuropeptides such as pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART). It is theorized that direct activation of GLP-1 receptors on POMC/CART neurons might induce satiety and indirectly inhibit the release of hunger-stimulating peptides neuropeptide Y (NPY) and agouti-related peptide (AgRP). Research also indicates that activation of GLP-1 receptors by agonists like Retatrutide may maintain elevated levels of free leptin and peptide YY3-36 (PYY3-36) during the reduction of weight.[8]
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- Folli F, Finzi G, Manfrini R, Galli A, Casiraghi F, Centofanti L, Berra C, Fiorina P, Davalli A, La Rosa S, Perego C, Higgins PB. Mechanisms of action of incretin receptor based dual- and tri-agonists in pancreatic islets. Am J Physiol Endocrinol Metab. 2023 Nov 1;325(5):E595-E609. doi: 10.1152/ajpendo.00236.2023. Epub 2023 Sep 20. PMID: 37729025; PMCID: PMC10874655. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10874655/
- Jakubowska A, Roux CWL, Viljoen A. The Road towards Triple Agonists: Glucagon-Like Peptide 1, Glucose-Dependent Insulinotropic Polypeptide and Glucagon Receptor – An Update. Endocrinol Metab (Seoul). 2024 Feb;39(1):12-22. doi: 10.3803/EnM.2024.1942. Epub 2024 Feb 14. PMID: 38356208; PMCID: PMC10901658. https://pubmed.ncbi.nlm.nih.gov/38356208/
- Doggrell SA. Is retatrutide (LY3437943), a GLP-1, GIP, and glucagon receptor agonist a step forward in the treatment of diabetes and obesity? Expert Opin Investig Drugs. 2023 May;32(5):355-359. doi: 10.1080/13543784.2023.2206560. Epub 2023 Apr 24. PMID: 37086147. https://pubmed.ncbi.nlm.nih.gov/37086147/
- Rosenstock J, Frias J, Jastreboff AM, Du Y, Lou J, Gurbuz S, Thomas MK, Hartman ML, Haupt A, Milicevic Z, Coskun T. Retatrutide, a GIP, GLP-1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double-blind, placebo and active-controlled, parallel-group, phase 2 trial conducted in the USA. Lancet. 2023 Aug 12;402(10401):529-544. doi: 10.1016/S0140-6736(23)01053-X Epub 2023 Jun 26. PMID: 37385280. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(23)01053-X/abstract
- Jastreboff AM, Kaplan LM, Frías JP, Wu Q, Du Y, Gurbuz S, Coskun T, Haupt A, Milicevic Z, Hartman ML; Retatrutide Phase 2 Obesity Trial Investigators. Triple-Hormone-Receptor Agonist Retatrutide for Obesity – A Phase 2 Trial. N Engl J Med. 2023 Aug 10;389(6):514-526. doi: 10.1056/NEJMoa2301972. Epub 2023 Jun 26. PMID: 37366315. https://pubmed.ncbi.nlm.nih.gov/37366315/
- Samms RJ, Sloop KW, Gribble FM, Reimann F, Adriaenssens AE. GIPR Function in the Central Nervous System: Implications and Novel Perspectives for GIP-Based Therapies in Treating Metabolic Disorders. Diabetes. 2021 Sep;70(9):1938-1944. doi: 10.2337/dbi21-0002. Epub 2021 Jun 27. PMID: 34176786; PMCID: PMC8576420. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8576420/
- Zhao X, Wang M, Wen Z, Lu Z, Cui L, Fu C, Xue H, Liu Y, Zhang Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front Endocrinol (Lausanne). 2021 Aug 23;12:721135. doi: 10.3389/fendo.2021.721135. PMID: 34497589; PMCID: PMC8419463. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8419463/
- Ard J, Fitch A, Fruh S, Herman L. Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Adv Ther. 2021 Jun;38(6):2821-2839. doi: 10.1007/s12325-021-01710-0. Epub 2021 May 11. PMID: 33977495; PMCID: PMC8189979. https://pubmed.ncbi.nlm.nih.gov/33977495/