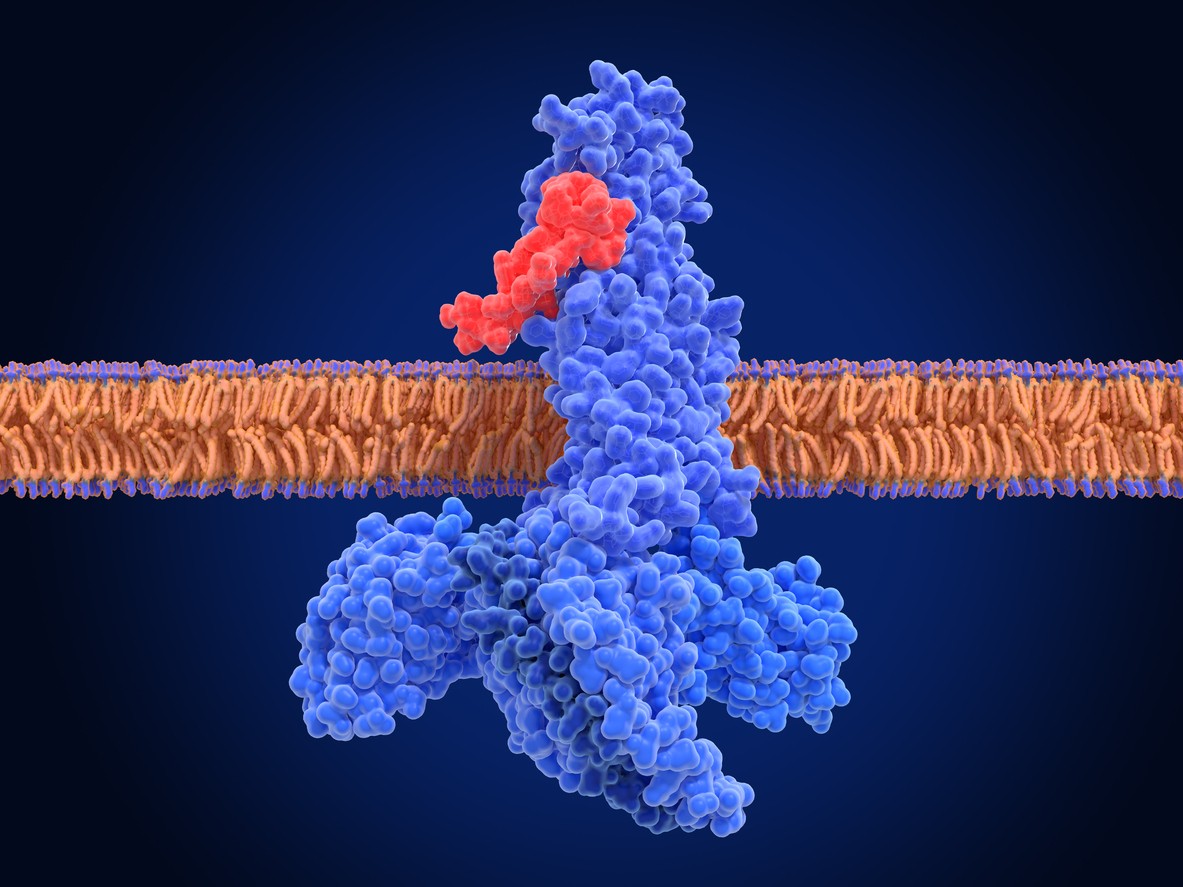
Like the endogenous GLP-1 peptide, Semaglutide, as a GLP-1 receptor agonist, has been researched for its potential to reduce blood glucose levels and decrease appetite.[1] Glucagon-like peptide-1 (GLP-1) is an incretin hormone produced in the intestines, primarily in response to nutrient ingestion. Research suggests that it plays a critical role in the regulation of glucose homeostasis, particularly in postprandial (after caloric intake) conditions. GLP-1 appears to stimulate insulin secretion from pancreatic beta cells in a glucose-dependent manner, potentially facilitating cellular glucose uptake and contributing to the reduction of blood glucose levels.
Mechanisms of Action
Studies suggest that there are two main ways through which the peptide may function:
Endogenous GLP-1: Endogenous GLP-1 is secreted following food intake. It slows gastric emptying, mitigates hunger hormone signals, and promotes insulin release when blood glucose levels are elevated. This physiological process aids in both glucose and weight regulation by reducing appetite and caloric intake.
GLP-1 Receptor Agonists: Synthetic GLP-1 receptor agonists have been developed in the course of diabetes research. These compounds appear to mimic the impacts of endogenous GLP-1 by activating its receptors on pancreatic beta cells, thereby increasing insulin secretion. They may also suppress glucagon release, further aiding in blood glucose regulation. Additionally, these agonists are considered to delay gastric emptying and mitigate hunger hormone signals, making them relevant to researchers studying the reduction of adipose tissue in laboratory models of type 2 diabetes.
The additional mechanism pathways include:
- Through binding with the GLP-1 receptors, Semaglutide may promote insulin secretion, i.e., glucose-dependent insulin release.[2]
- Semaglutide is thought to support pancreatic beta cell function, supporting the proinsulin-to-insulin ratio.[3]
- By delaying gastric emptying and reducing appetite, the compound may contribute to weight reduction.[4]
Scientific and Research Studies
Semaglutide (GLP-1) Peptide and the Incretin Effect
Research suggests that the Semaglutide (GLP-1) peptide theoretically displays ‘the incretin effect.’ ‘The Incretin Effect’ is a physiological response mediated by gastrointestinal hormones released post-caloric intake to reduce blood glucose levels.
GLP-1 receptors located on pancreatic beta cells appear to bind with Semaglutide. This is intended to stimulate insulin secretion and aid in glucose control.
According to J.J. Holst, “The main actions of GLP-1 are to stimulate insulin secretion (i.e., to act as an incretin hormone) and to inhibit glucagon secretion, thereby contributing to limit postprandial glucose excursions.”[1] As a GLP-1 receptor agonist, Semaglutide appears to promote incretin hormone activity and, therefore, may have a hand in the regulation of blood sugar levels.
Semaglutide (GLP-1) Peptide and Pancreatic Beta Cell Protection
Research suggests that Semaglutide (GLP-1) peptide has the potential to protect pancreatic beta cells, as seen in a study[5] conducted on non-obese diabetic (NOD) mice models. In this experiment, mice were introduced to Semaglutide in combination with lisofylline, an immunomodulatory agent that suppresses autoimmune activity, and exendin-4, a compound regarded for its proposed ability to promote beta cell proliferation. Based on the results, it appeared that Semaglutide might stimulate pancreatic beta-cell growth and mitigate beta-cell apoptosis (cell death).
Furthermore, results also suggested that the mice maintained optimal glucose levels up to 145 days post-introduction, even after discontinuation of the Semaglutide (GLP-1) peptide. Because of these long-lasting impacts, it is speculated that Semaglutide may have enduring relevance to glucose regulation and beta cell preservation.
Semaglutide (GLP-1) Peptide and Appetite Suppression
GLP-1 receptor agonists, including Semaglutide, appear to impact appetite regulation through the delay of gastric motility. This potentially leads to a prolonged feeling of fullness, thereby reducing overall food intake.[1] Research[6] suggests that Semaglutide, when introduced in the brain, may reduce the neural drive to consume calories, possibly leading to a decrease in appetite and better-supporting mitigation of hunger hormone signals.
Experimental studies in murine models suggest that Semaglutide’s central action curtails food intake, potentially making it an agent in the reduction of excessive adipose tissue. It is also speculated that over time, Semaglutide may result in gradual weight reduction, possibly contributing to better-supported cardiovascular function and increased energy levels.
Semaglutide (GLP-1) Peptide and Neurological Research
Semaglutide, through its action on GLP-1 receptors (GLP-1R), is suggested to possess neuroprotective and cognition-supporting properties. GLP-1 and its receptors are expressed in brain cells, and deficiencies in GLP-1R are associated with seizures, impaired learning, and neuronal damage. Research by Mathew J. During et al. highlights that “Systemic administration of GLP-1 receptor agonists in wild-type animals [may] prevent kainate-induced apoptosis of hippocampal neurons. Brain GLP-1R represents a promising new target for cognitive-enhancing and neuroprotective agents.”[7]
Additionally, studies[8] have posited that Semaglutide (GLP-1) peptide may protect hippocampal regions of the brain from cellular apoptosis, indicating their potential in addressing neurodegenerative conditions such as Alzheimer’s disease. Furthermore, Semaglutide also appears to reduce beta-amyloid concentrations, which are thought to be linked to the development of Alzheimer’s. Some research posits a possibility that the peptide may contribute to the delay or reversal of some symptoms of neurodegeneration.
Semaglutide (GLP-1) Peptide and Cardiovascular Function
Studies suggest that GLP-1 receptors are distributed throughout the cardiovascular system, where their activation appears to play a crucial role in maintaining cardiac function.[9] It appears that Semaglutide, through its theorized action on GLP-1 receptors, may help regulate blood pressure and reduce left ventricular diastolic pressure, both of which are deemed essential in mitigating cardiac hypertrophy and related cardiovascular complications.
Additionally, Semaglutide (GLP-1) peptide is speculated to support glucose uptake in muscle cells specific to muscular tissue in the cardiovascular system, particularly in ischemic or weakened myocardial tissue following a heart attack. Better-supported glucose metabolism appears to further support cardiac function and may aid in reversing the adverse impacts of myocardial infarction.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide is a glucagon-like peptide-1 receptor agonist with cardiovascular benefits for the management of type 2 diabetes. Rev Endocr Metab Disord. 2022 Jun;23(3):521-539. doi: 10.1007/s11154-021-09699-1. Epub 2022 Jan 7. PMID: 34993760; PMCID: PMC8736331. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8736331/#:~:text=Semaglutide%20improves%20the%20efficiency%20of,as%20postprandial%20glucose%20%5B26%5D
- Knudsen LB, Lau J. The Discovery and Development of Liraglutide and Semaglutide. Front Endocrinol (Lausanne). 2019 Apr 12;10:155. doi: 10.3389/fendo.2019.00155. PMID: 31031702; PMCID: PMC6474072. https://pubmed.ncbi.nlm.nih.gov/31031702/
- Ahmann AJ, Capehorn M, Charpentier G, Dotta F, Henkel E, Lingvay I, Holst AG, Annett MP, Aroda VR. Efficacy and Safety of Once-Weekly Semaglutide Versus Exenatide ER in Subjects With Type 2 Diabetes (SUSTAIN 3): A 56-Week, Open-Label, Randomized Clinical Trial. Diabetes Care. 2018 Feb;41(2):258-266. doi: 10.2337/dc17-0417. Epub 2017 Dec 15. PMID: 29246950. https://pubmed.ncbi.nlm.nih.gov/29246950/
- Christou GA, Katsiki N, Blundell J, Fruhbeck G, Kiortsis DN. Semaglutide is a promising anti-obesity drug. Obes Rev. 2019 Jun;20(6):805-815. doi: 10.1111/obr.12839. Epub 2019 Feb 15. PMID: 30768766. https://pubmed.ncbi.nlm.nih.gov/30768766/
- Yang Z, Chen M, Carter JD, Nunemaker CS, Garmey JC, Kimble SD, Nadler JL. Combined treatment with lisofylline and exendin-4 reverses autoimmune diabetes. Biochem Biophys Res Commun. 2006 Jun 9;344(3):1017-22. doi: 10.1016/j.bbrc.2006.03.177. Epub 2006 Apr 5. PMID: 16643856. https://pubmed.ncbi.nlm.nih.gov/16643856/
- Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH, Taylor KL, Trautmann ME, Kim DD, Kendall DM. Interim analysis of the effects of exenatide treatment on A1C, weight, and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006 Jul;8(4):436-47. doi: 10.1111/j.1463-1326.2006.00602.x. PMID: 16776751. https://pubmed.ncbi.nlm.nih.gov/16776751/
- During MJ, Cao L, Zuzga DS, Francis JS, Fitzsimons HL, Jiao X, Bland RJ, Klugmann M, Banks WA, Drucker DJ, Haile CN. The glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat Med. 2003 Sep;9(9):1173-9. doi: 10.1038/nm919. Epub 2003 Aug 17. PMID: 12925848. https://pubmed.ncbi.nlm.nih.gov/12925848/
- Perry T, Haughey NJ, Mattson MP, Egan JM, Greig NH. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J Pharmacol Exp Ther. 2002 Sep;302(3):881-8. doi: 10.1124/jpet.102.037481. PMID: 12183643. https://pubmed.ncbi.nlm.nih.gov/12183643/
- Gros R, You X, Baggio LL, Kabir MG, Sadi AM, Mungrue IN, Parker TG, Huang Q, Drucker DJ, Husain M. Cardiac function in mice lacking the glucagon-like peptide-1 receptor. Endocrinology. 2003 Jun;144(6):2242-52. doi: 10.1210/en.2003-0007. PMID: 12746281. https://pubmed.ncbi.nlm.nih.gov/12746281/