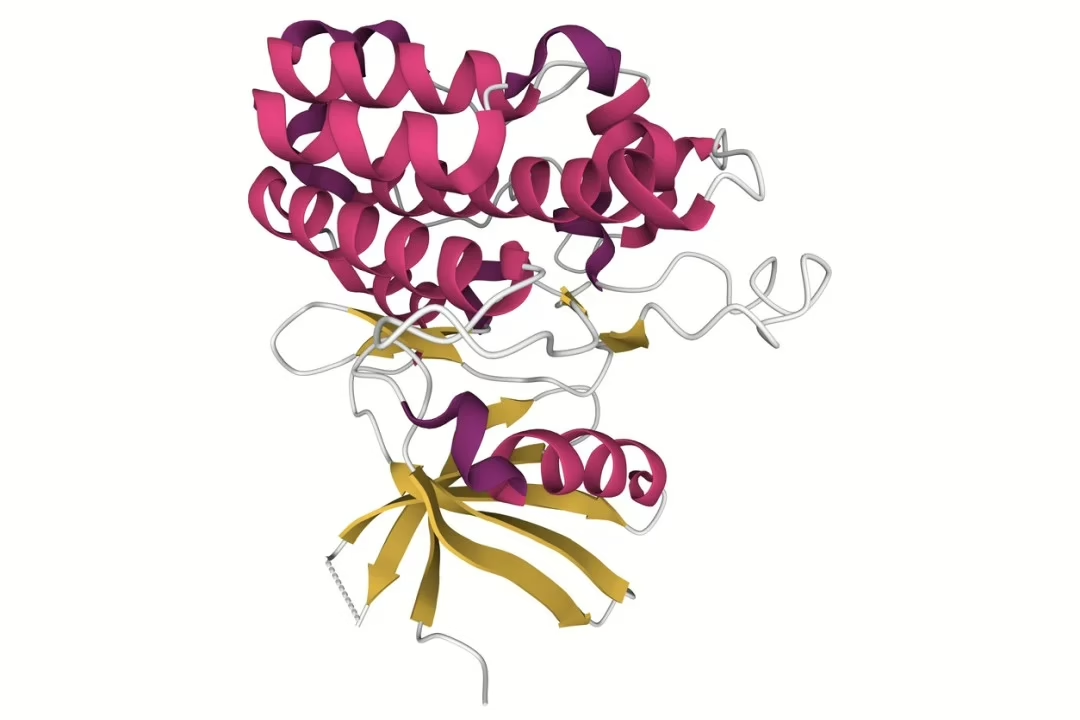
Research suggests that Follistatin exists primarily in two isoforms, FST-317 and FST-344, with 288 and 315 amino acids, respectively. These isoforms arise through alternative mRNA splicing, resulting in distinct molecular structures.[1] Among these, FST-344 is regarded as the predominant form, while FST-317 represents a smaller proportion of the total encoded mRNA.
FST-344 consists of three highly conserved domains—FSD1, FSD2, and FSD3—comprising approximately 63 amino acid residues each, with structural conservation maintained in synthetic derivatives.[2] These domains contain 73–77 amino acid residues, including 10 conserved cysteine residues, which contribute to protein stability and functionality. Due to its diverse regulatory roles, FST-344 has been extensively investigated for its interactions with various members of the Transforming Growth Factor-beta (TGF-β) superfamily.
Mechanism of Action
The primary function of FST-344 is hypothesized to involve binding and inhibition of activins, proteins within the TGF-β superfamily that regulate reproductive and cellular processes.[3] Activins, particularly those secreted by ovarian follicles, enhance the secretion of follicle-stimulating hormone (FSH), and a critical regulator of reproductive physiology. FST-344 has been suggested to mitigate activin-mediated FSH secretion by forming high-affinity inhibitory complexes, thus modulating endocrine function.
FST-344 is believed to be synthesized locally within the pituitary gland, gonads, testes, and ovaries in certain organisms, although it has also been detected in the bloodstream, suggesting potential systemic roles beyond reproductive function. In addition to its interaction with activins, FST-344 is hypothesized to bind to other TGF-β family proteins, including Bone Morphogenetic Proteins (BMPs), which are implicated in bone formation, embryogenesis, and cellular differentiation. However, the precise role of FST-344 in BMP regulation remains an area of ongoing investigation.[4]
One of the most studied interactions of FST-344 involves Growth Differentiation Factor 8 (GDF8), commonly referred to as myostatin. Myostatin functions as a negative regulator of muscle growth by limiting myocyte proliferation and differentiation. It is proposed that FST-344 binds to myostatin, potentially neutralizing its inhibitory effects and facilitating increased muscle mass. This myostatin inhibition has been widely explored in the context of skeletal muscle physiology, with research suggesting that FST-344-mediated suppression of myostatin might promote muscle hypertrophy. However, the extent of this effect and its underlying mechanisms remain subject to further scientific scrutiny.
Additionally, FST-344 may interact with Growth Differentiation Factor 9 (GDF9), a protein essential for ovarian follicle development. Preliminary findings suggest that FST-344 could play a role in regulating GDF9 activity, though this interaction remains incompletely characterized. Given its broad functional scope, further research is required to elucidate the full spectrum of FST-344’s biological roles, particularly concerning its interactions within the TGF-β superfamily.
Scientific Research and Studies
FST-344 and Breast Cancer Progression
Studies employing reverse transcription polymerase chain reaction (RT-PCR) analysis have suggested that Follistatin expression may vary in breast cancer models.[5]
A study examining gene expression datasets in murine models of breast cancer reported that Follistatin was frequently under-expressed in malignant breast cells.[6] This downregulation is hypothesized to contribute to enhanced cancer cell proliferation, potentially mediated by activin proteins. Given that Follistatin has been proposed to bind and inhibit activins, researchers speculate that restoring Follistatin levels might attenuate activin-induced metastasis and improve overall survival outcomes. However, further investigations are required to elucidate the precise regulatory mechanisms of Follistatin in breast cancer progression.
FST-344 and Esophageal Carcinogenesis
Bone morphogenetic proteins (BMPs) have been implicated in the pathological transformation of normal esophageal epithelial cells into malignant phenotypes. Follistatin, hypothesized to exhibit binding affinity for activin and myostatin, is also suggested to interact with BMPs, potentially modulating their activity. By regulating BMP signaling, Follistatin might mitigate excessive cellular proliferation, a hallmark of oncogenic processes. Experimental findings on FST-344 suggest that this peptide may counteract the effects of acid reflux, considered to be a contributor to esophageal tissue inflammation and carcinogenesis. Specifically, Follistatin’s ability to inhibit BMP activity could theoretically interfere with the initiation of malignant transformation, particularly in microenvironments predisposed to chronic inflammation or external stressors such as prolonged acid exposure.[7]
While preliminary data suggest a possible protective role, further research is necessary to determine the mechanistic pathways underlying this interaction.
FST-344 and Skeletal Muscle Modulation
Myostatin, a member of the transforming growth factor-beta (TGF-β) superfamily, is widely recognized for its role in regulating muscle mass by inhibiting muscle cell proliferation and differentiation. As a key regulator of muscle homeostasis, myostatin is primarily synthesized by muscle cells and functions as a negative modulator of excessive muscle growth. FST-344, a glycoprotein that may counteract myostatin activity, has been explored for its potential influence on muscle physiology.
Studies suggest that FST-344 may disrupt myostatin signaling, thereby promoting muscle growth. Based on the study conducted in 1997,[8] mice subjected to FST-344 exhibited significantly lower myostatin expression. This reduction appeared to correlate with enhanced skeletal muscle mass, with subjects indicating both muscle hypertrophy and hyperplasia, leading to a substantial increase in body mass compared to controls.
Further research has investigated the possibility of endogenously producing FST-344 via mRNA-based delivery systems. In one study, a nanoparticle-mediated mRNA approach was used to stimulate hepatic cells to synthesize and release Follistatin. The results suggested that within 72 hours of peptide exposure, circulating Follistatin levels were elevated, which coincided with a decrease in myostatin and activin A concentrations.[9]
Activin A, another TGF-β superfamily member, is implicated in various cellular processes, including differentiation, proliferation, and apoptosis. It has been associated with muscle atrophy through its interaction with the activin type IIB receptor (ActRIIB), which appears to activate pathways that promote muscle protein degradation. Research findings suggest that prolonged elevations in Follistatin levels over an eight-week period resulted in a 10% increase in lean muscle mass compared to untreated controls.
Unlike research focused solely on myostatin inhibition—such as studies using anti-myostatin antibodies—Follistatin-344 is hypothesized to exert broader effects by modulating both myostatin and activin A pathways. While myostatin inhibition primarily facilitates muscle hypertrophy, activin A suppression may mitigate additional contributors to muscle loss, including fibrosis and inflammatory responses. This proposed dual mechanism of action may provide a more comprehensive approach to muscle function by enhancing muscle mass while simultaneously improving muscle quality and function, potentially reducing fibrosis-related stiffness and muscle weakness.[10]
FST-344 and Liver Fibrosis
A study was conducted to examine the potential role of Follistatin in mitigating liver fibrosis, specifically focusing on its impact in the early stages of the condition.
In this investigation,[11] rats were assigned to either a control group or a Follistatin-exposed group for a duration of four weeks. The results suggested that the Follistatin-exposed group exhibited a significant 32% reduction in liver fibrosis compared to the control. Additionally, a substantial decrease in hepatocytic apoptosis—approximately 90%—was observed in the Follistatin-exposed animals, suggesting a protective effect against liver cellular damage.
FST-344 and Hair Follicle Growth
FST-344 has been explored for its potential role in promoting tissue regeneration, particularly in relation to hair follicle stimulation and hair growth.
A study[12] studying the potential of a synthetic Follistatin-based formulation, referred to as Hair Stimulating Complex (HSC), was conducted on research models of hair loss. The study examined 26 models exposed to the peptide over the course of 52 weeks. Histopathological analyses indicated a notable improvement in hair follicle growth within the peptide-exposed group when compared to controls. In addition to promoting hair follicle growth, the peptide-exposed research models indicated an increase in hair thickness and density, with a reported improvement of approximately 13%.
FST-344 and Cell Proliferation, Metastasis Regulation
Research investigating the potential of Follistatin on breast cancer suggested an intriguing dual role, wherein Follistatin potentially promotes cell proliferation while simultaneously inhibiting metastasis. This dichotomy appears to extend across various tissues, including the liver, where Follistatin is speculated to play a critical role in hepatocyte proliferation. Studies using rat models suggest that the inactivation of activin, mediated by Follistatin, is considered essential for the initiation of hepatocyte proliferation.[13]
This observation offers insight into the dual role of Follistatin, which is often linked to increased tumor growth while concurrently limiting tumor invasion and metastasis. It is hypothesized that during cellular growth, an energy trade-off occurs, wherein migratory functions are inhibited to prioritize cellular resources for growth and proliferation. This mechanism may account for the potential of Follistatin in modulating both tumor growth and its dissemination.
FST-344 and Insulin Deficiency
Research in murine models suggests that overexpression of FST-344 may significantly impact insulin regulation by increasing the mass of beta-islet cells, which are considered to be responsible for the production of insulin. This enhancement in islet cell mass may result in improved insulin secretion and better regulation of blood glucose levels. In particular, studies have reported that Follistatin exposure in mice may lead to reduced fasting glucose levels and a marked reduction in certain symptoms associated with diabetes.
Per the study reports, the peptide-exposed mice exhibited a substantial improvement in longevity, with their lifespans doubling compared to non-peptide-exposed counterparts. This increase appears to be attributed to the virtual elimination of diabetes-related complications, suggesting that Follistatin may potentially play a critical role in ameliorating the long-term effects of both Type 1 and Type 2 diabetes. By supporting the function of the remaining functional islet cells within the pancreas, Follistatin may offer a potential mode to restore some level of insulin production and function, even in the presence of insulin resistance or autoimmune destruction of beta cells.[14]
This reported mechanism might provide an alternative to the traditional study of exogenous insulin in diabetes research. Unlike insulin, which only supplements insulin levels, Follistatin’s action is suggested to work within internal physiological controls. By boosting endogenous insulin secretion, it could potentially offer a more natural and finely regulated approach to managing blood sugar levels. As per researchers, the studies suggest “overexpression of FST in the diabetic pancreas preserves β-cell function by promoting β-cell proliferation.”
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- FST follistatin [Homo sapiens (human)]. https://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=DetailsSearch&Term=10468
- Shi, L., Resaul, J., Owen, S., Ye, L., & Jiang, W. G. (2016). Clinical and Therapeutic Implications of Follistatin in Solid Tumours. Cancer genomics & proteomics, 13(6), 425–435. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5219916/
- Rodino-Klapac, L. R., Haidet, A. M., Kota, J., Handy, C., Kaspar, B. K., & Mendell, J. R. (2009). Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle & nerve, 39(3), 283–296. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2717722/
- Reichel C, Gmeiner G, Thevis M. Detection of black market follistatin 344. Drug Test Anal. 2019 Nov;11(11-12):1675-1697. doi: 10.1002/dta.2741. Erratum in: Drug Test Anal. 2020 Oct;12(10):1522-1533. doi: 10.1002/dta.2882. PMID: 31758732. https://pubmed.ncbi.nlm.nih.gov/31758732/
- Zabkiewicz C, Resaul J, Hargest R, Jiang WG, Ye L. Increased Expression of Follistatin in Breast Cancer Reduces Invasiveness and Clinically Correlates with Better Survival. Cancer Genomics Proteomics. 2017 Jul-Aug;14(4):241-251. https://pubmed.ncbi.nlm.nih.gov/28647698/
- Seachrist DD, Sizemore ST, Johnson E, Abdul-Karim FW, Weber Bonk KL, Keri RA. Follistatin is a metastasis suppressor in a mouse model of HER2-positive breast cancer. Breast Cancer Res. 2017 Jun 5;19(1):66. doi: 10.1186/s13058-017-0857-y. PMID: 28583174; PMCID: PMC5460489. https://pubmed.ncbi.nlm.nih.gov/28583174/
- Lau MC, Ng KY, Wong TL, Tong M, Lee TK, Ming XY, Law S, Lee NP, Cheung AL, Qin YR, Chan KW, Ning W, Guan XY, Ma S. FSTL1 Promotes Metastasis and Chemoresistance in Esophageal Squamous Cell Carcinoma through NFκB-BMP Signaling Cross-talk. Cancer Res. 2017 Nov 1. https://pubmed.ncbi.nlm.nih.gov/28883005/
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997 May 1;387(6628):83-90. https://pubmed.ncbi.nlm.nih.gov/9139826/
- Schumann C, Nguyen DX, Norgard M, Bortnyak Y, Korzun T, Chan S, Lorenz AS, Moses AS, Albarqi HA, Wong L, Michaelis K, Zhu X, Alani AWG, Taratula OR, Krasnow S, Marks DL, Taratula O. Increasing lean muscle mass in mice via nanoparticle-mediated hepatic delivery of follistatin mRNA. Theranostics 2018; 8(19):5276-5288. doi:10.7150/thno.27847. https://www.thno.org/v08p5276.htm
- Iskenderian A, Liu N, Deng Q, Huang Y, Shen C, Palmieri K, Crooker R, Lundberg D, Kastrapeli N, Pescatore B, Romashko A, Dumas J, Comeau R, Norton A, Pan J, Rong H, Derakhchan K, Ehmann DE. Myostatin and activin blockade by engineered follistatin results in hypertrophy and improves dystrophic pathology in mdx mouse more than myostatin blockade alone. Skelet Muscle. 2018 Oct 27;8(1):34. https://pubmed.ncbi.nlm.nih.gov/30368252/
- Patella S, Phillips DJ, Tchongue J, de Kretser DM, Sievert W. Follistatin attenuates early liver fibrosis: effects on hepatic stellate cell activation and hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2006 Jan;290(1):G137-44. https://pubmed.ncbi.nlm.nih.gov/16123203/
- Zimber MP, Ziering C, Zeigler F, Hubka M, Mansbridge JN, Baumgartner M, Hubka K, Kellar R, Perez-Meza D, Sadick N, Naughton GK. Hair regrowth following a Wnt- and follistatin containing treatment: safety and efficacy in a first-in-man phase 1 clinical trial. J Drugs Dermatol. 2011 Nov;10(11):1308-12. https://pubmed.ncbi.nlm.nih.gov/22052313/
- Ooe H, Chen Q, Kon J, Sasaki K, Miyoshi H, Ichinohe N, Tanimizu N, Mitaka T. Proliferation of rat small hepatocytes requires follistatin expression. J Cell Physiol. 2012 Jun;227(6):2363-70. doi: 10.1002/jcp.22971. PMID: 21826650. https://pubmed.ncbi.nlm.nih.gov/21826650/
- Zhao C, Qiao C, Tang RH, Jiang J, Li J, Martin CB, Bulaklak K, Li J, Wang DW, Xiao X. Overcoming Insulin Insufficiency by Forced Follistatin Expression in β-cells of db/db Mice. Mol Ther. 2015 May;23(5):866-874. doi: 10.1038/mt.2015.29. Epub 2015 Feb 13. PMID: 25676679; PMCID: PMC4427879. https://pubmed.ncbi.nlm.nih.gov/25676679/