Contents:
- Tesamorelin Peptide
- CJC-1295 (Mod GRF 1-29) Peptide
- Ipamorelin Peptide
- Scientific and Research Studies
- Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin Blend and the Pituitary Gland
- Tesamorelin, CJC-1295 (Mod GRF 1-29)F, and Ipamorelin and Cardiovascular Action
- Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin and Effect on Gastrointestinal Tract
- Synergistic Potential of Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin Peptides
- References
Tesamorelin Peptide
Tesamorelin is a synthetic analog of growth hormone-releasing hormone (GHRH), engineered to resemble the natural peptide that scientists consider to be responsible for stimulating GH secretion. Structurally, Tesamorelin consists of 44 amino acids and incorporates specific modifications to enhance its stability against enzymatic degradation. One notable modification is the introduction of a trans-3-hexenoic acid group at its C-terminus, often referred to as an omega-amino acid modification.[1] This alteration is hypothesized to improve resistance to enzymatic breakdown, prolonging its functional lifespan in biological systems. Additionally, the N-terminal acetylation (CH₃CO-) has been suggested to further contribute to its structural resilience and bioactivity. These modifications result in the designation N-(trans-3-hexenoyl)-[Tyr1]hGRF(1–44)NH2 acetate.
Tesamorelin’s mechanism of action is thought to involve binding to GHRH receptors located in the hypothalamus and pituitary gland,[1] thereby triggering the release of endogenous GH. This proposed mechanism has garnered interest in exploring its implications for protein metabolism, lipid oxidation, and cellular growth processes.
CJC-1295 (Mod GRF 1-29) Peptide
CJC-1295 (Mod GRF 1-29) is a synthetic derivative of the functional segment of GHRH, comprising 29 amino acids. This peptide is often described as a tetrasubstituted variant designed to resist enzymatic degradation and extend its half-life. Unlike its predecessor with a Drug Affinity Complex (DAC), the Mod GRF 1-29 variant lacks the DAC but appears to have retained significant stability and activity. Research suggests that CJC-1295 interacts with GHRH receptors in the pituitary gland, where it may stimulate GH secretion.
Its proposed mechanism mimics that of GHRH, potentially enhancing protein synthesis, promoting muscle hypertrophy, and influencing metabolic pathways.[2] The peptide’s engineered design aims to maintain functionality while reducing susceptibility to degradation, making it a key component of this peptide blend.
Ipamorelin Peptide
Ipamorelin is a synthetic pentapeptide and a selective agonist for the ghrelin receptor, also known as the growth hormone secretagogue (GHS) receptor. This receptor is predominantly expressed in the pituitary gland, where its activation may stimulate the release of GH. Ipamorelin exhibits high specificity for the GHS receptor, with minimal interaction with other hormonal systems, reducing the likelihood of off-target action.
Research suggests that Ipamorelin’s apparent selective binding to the GHS receptor might enhance GH secretion without altering levels of other hormones, such as cortisol or prolactin.[3] This unique mechanism positions Ipamorelin as a potential tool for investigating GH-related physiological processes, including growth, repair, and metabolic regulation.
This tri-peptide blend appears to combine Tesamorelin’s GHRH receptor affinity, CJC-1295’s enhanced stability and activity, and Ipamorelin’s selective ghrelin receptor stimulation. Together, these peptides may offer synergistic effects on GH secretion, which warrants further investigation through controlled research studies.
Scientific Research and Studies
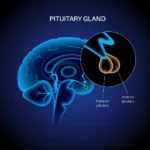
Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin Blend and the Pituitary Gland
The peptide blend comprising Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin is speculated to potentially interact with the pituitary gland, primarily through receptor-specific binding that may modulate the secretion of growth hormone (GH).
Tesamorelin and CJC-1295 (Mod GRF 1-29) are analogs of growth hormone-releasing hormone (GHRH) that appear to target GHRH receptors on somatotrophs in the anterior pituitary. Research suggests that their interaction may induce receptor conformational changes, activating downstream intracellular signaling cascades.[4] Specifically, these peptides are hypothesized to stimulate adenylate cyclase, leading to the conversion of adenosine triphosphate (ATP) into cyclic adenosine monophosphate (cAMP). The resulting elevation of cAMP levels is thought to activate protein kinase A (PKA), which phosphorylates various intracellular targets. This signaling cascade is believed to culminate in the synthesis and secretion of human growth hormone (hGH). The hGH released from somatotroph cells may also facilitate the production of insulin-like growth factor-1 (IGF-1), deemed a critical mediator of growth hormone activity.
CJC-1295 (Mod GRF 1-29) exhibits notable structural modifications, including four amino acid substitutions, which may enhance its resistance to enzymatic degradation. This increased stability is reported to extend its half-life, improving its pharmacokinetic profile. Additionally, these modifications allow a proportion of the peptide to covalently bind to serum albumin, thereby prolonging its duration of action. Trace binding to fibrinogen and immunoglobulin G (IgG) has also been observed. These properties collectively suggest a sustained mechanism of action, with a potential for increased biological activity.[5]
Ipamorelin, in contrast, is a selective agonist for the ghrelin receptor, also referred to as the growth hormone secretagogue receptor (GHSR). This peptide is believed to bind to GHSR on somatotroph cells, initiating signaling pathways that may promote GH secretion. Unlike some other secretagogues, Ipamorelin appears to exert a high degree of specificity for GHSR, with apparently minimal off-target effects on other endocrine pathways, including prolactin and adrenocorticotropic hormone (ACTH) regulation.[6]
In vitro studies[7] suggest that Ipamorelin binding to GHSR activates phospholipase C (PLC), which catalyzes the generation of inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 may mobilize calcium ions (Ca2+) from intracellular stores, while DAG is thought to activate protein kinase C (PKC). These intracellular events potentially facilitate the exocytosis of vesicles containing GH from somatotroph cells. This process underscores the peptide’s role in GH secretion through precise intracellular signaling mechanisms.
The combined properties of Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin suggest a synergistic effect on pituitary function. Tesamorelin and CJC-1295 (Mod GRF 1-29) focus on GHRH receptor pathways, while Ipamorelin appears to activate the GHSR, providing complementary mechanisms to stimulate GH release. These peptides collectively indicate complex interactions with the pituitary gland, potentially enhancing GH secretion through distinct yet interconnected molecular pathways. Such findings highlight their potential implications in regulating growth hormone activity and associated metabolic processes. Further research is necessary to elucidate the full scope of their physiological effects and research applications.
Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin and Cardiovascular Action
The potential cardiovascular effects of Tesamorelin, Modified GRF (CJC-1295), and Ipamorelin have been studied with an emphasis on cardiac repair and lipid metabolism. Following myocardial infarction (MI), cardiac tissue often undergoes scarring, which can impair heart function, including ejection fraction and contractility. Research in animal models suggests that growth hormone secretagogues may possibly enhance post-MI cardiac repair. Observed outcomes include reduced infarct size, improved cardiac ejection fraction, and restoration of overall cardiac function.[8]
Ipamorelin’s activation of the GHS-R1a receptor is hypothesized to exert a possible cardioprotective effect through positive inotropic properties. Researchers elucidate that, “Through activation of GHS-R1a, secretagogues produced a positive inotropic effect on ischemic cardiomyocytes and protected them from I/R injury, likely by safeguarding or restoring p-PLB (and hence SR Ca2+ content) to facilitate the maintenance or recovery of normal cardiac contractility.”
Additionally, Tesamorelin, while primarily recognized for its lipodystrophy-reducing properties, has exhibited potential cardiovascular action, particularly in HIV-positive models. Some studies highlight its potential to lower triglycerides, total cholesterol, and non-HDL cholesterol levels,[9] suggesting a broader impact on lipid metabolism and cardiovascular function. These findings collectively underscore the potential of this peptide blend to modulate cardiac function and lipid profiles.
Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin and Effect on Gastrointestinal Tract
The peptide blend comprising Tesamorelin, Modified GRF (CJC-1295), and Ipamorelin may hold varying degrees of influence on the gastrointestinal (GI) tract through distinct mechanisms. Tesamorelin appears to enhance gastric emptying and gastrointestinal motility, as observed in preclinical studies. This action suggests its potential utility in addressing motility disorders or delayed gastric emptying.
Modified GRF, while exhibiting minimal direct effects on motility, appears to support GI function by improving gut barrier integrity and mitigating intestinal inflammation. This effect has been primarily studied in animal models of colitis, where the peptide appeared to host anti-inflammatory properties and potential contributions to the restoration of gut homeostasis.
Ipamorelin, as a selective agonist of the ghrelin receptor, is posited to exert effects through receptor activation within the GI tract. Upon binding to ghrelin receptors, Ipamorelin may stimulate gut motility, improve nutrient absorption, and contribute to tissue repair following gastrointestinal injury. Furthermore, preclinical research indicates its potential to mitigate inflammation and promote recovery in various models of GI injury. According to the studies, Ipamorelin may “increase total body fat percentages,” suggesting the peptide is a “potent and selective stimulator of GH that [may] significantly influence the GI system, body composition, and adiposity.”[10]
Synergistic Potential of Tesamorelin, CJC-1295 (Mod GRF 1-29), and Ipamorelin Peptides
The combination of Tesamorelin, Modified GRF (CJC-1295), and Ipamorelin has been proposed by its researchers to enhance growth hormone (GH) secretion through distinct yet complementary mechanisms of action. Studies suggest that Tesamorelin and Modified GRF, when combined, may produce a synergistic increase in GH levels, surpassing the effects of either peptide alone.[11] This blend is also speculated to cause reductions in visceral adipose tissue, particularly in models of HIV-associated lipodystrophy, though this effect may have been reversed upon study cessation.
Beyond GH secretion, this peptide combination, as per the research, is suggested to support improved sleep-wake cycles, increased behavioral mood, energy, and sustained GH production over time. Together, these peptides may also synergistically enhance insulin-like growth factor 1 (IGF-1) levels, potentially leading to improved muscle growth, bone density, cognitive function, and cellular repair, making this blend a promising tool for studies exploring diverse metabolic and physiological challenges.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 9831659, Ipamorelin. https://pubchem.ncbi.nlm.nih.gov/compound/Ipamorelin.
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 56841945. https://pubchem.ncbi.nlm.nih.gov/compound/56841945.
- National Center for Biotechnology Information (2023). PubChem Compound Summary for CID 16137828, Tesamorelin. https://pubchem.ncbi.nlm.nih.gov/compound/Tesamorelin
- Spooner, L. M., & Olin, J. L. (2012). Tesamorelin: a growth hormone-releasing factor analogue for HIV-associated lipodystrophy. The Annals of pharmacotherapy, 46(2), 240–247. https://doi.org/10.1345/aph.1Q629
- Zhou, F., Zhang, H., Cong, Z., Zhao, L. H., Zhou, Q., Mao, C., Cheng, X., Shen, D. D., Cai, X., Ma, C., Wang, Y., Dai, A., Zhou, Y., Sun, W., Zhao, F., Zhao, S., Jiang, H., Jiang, Y., Yang, D., Eric Xu, H., … Wang, M. W. (2020). Structural basis for activation of the growth hormone-releasing hormone receptor. Nature communications, 11(1), 5205. https://doi.org/10.1038/s41467-020-18945-0
- Sinha DK, Balasubramanian A, Tatem AJ, Rivera-Mirabal J, Yu J, Kovac J, Pastuszak AW, Lipshultz LI. Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Transl Androl Urol. 2020 Mar;9(Suppl 2):S149-S159. doi: 10.21037/tau.2019.11.30. PMID: 32257855; PMCID: PMC7108996 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108996/
- Jiménez-Reina, L., Cañete, R., de la Torre, M. J., & Bernal, G. (2002). Influence of chronic treatment with the growth hormone secretagogue Ipamorelin, in young female rats: somatotroph response in vitro. Histology and histopathology, 17(3), 707–714. https://doi.org/10.14670/HH-17.707
- Ma Y, Zhang L, Edwards JN, Launikonis BS, Chen C. Growth hormone secretagogues protect mouse cardiomyocytes from in vitro ischemia/reperfusion injury through regulation of intracellular calcium. PLoS One. 2012;7(4):e35265. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0035265 Epub 2012 Apr 6. PMID: 22493744; PMCID: PMC3320867. https://pmc.ncbi.nlm.nih.gov/articles/PMC3320867/
- Stanley TL, Falutz J, Marsolais C, Morin J, Soulban G, Mamputu JC, Assaad H, Turner R, Grinspoon SK. Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin. Clin Infect Dis. 2012 Jun;54(11):1642-51. doi: 10.1093/cid/cis251. Epub 2012 Apr 10. PMID: 22495074; PMCID: PMC3348954. https://pubmed.ncbi.nlm.nih.gov/22495074/
- Sinha DK, Balasubramanian A, Tatem AJ, Rivera-Mirabal J, Yu J, Kovac J, Pastuszak AW, Lipshultz LI. Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Transl Androl Urol. 2020 Mar;9(Suppl 2):S149-S159. doi: 10.21037/tau.2019.11.30. PMID: 32257855; PMCID: PMC7108996 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7108996/
- Bedimo R. Growth hormone and tesamorelin in the management of HIV-associated lipodystrophy. HIV AIDS (Auckl). 2011;3:69-79. doi: 10.2147/HIV.S14561. Epub 2011 Jul 10. PMID: 22096409; PMCID: PMC3218714. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3218714/