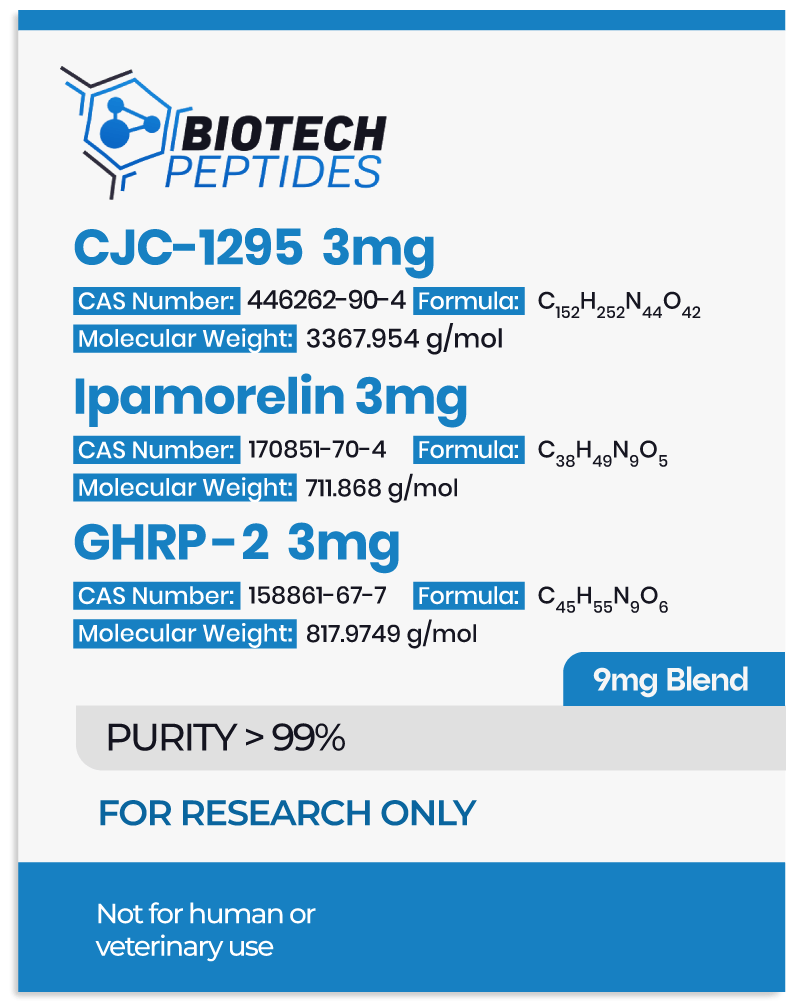
CJC-1295 belongs to a class of molecules known as growth hormone-releasing hormone (GHRH) agonists. It is a tetrasubstituted version of GHRH 1-29, representing the shortest functional sequence of GHRH. GHRH 1-29 consists of the first 29 amino acids of the native GHRH peptide and may potentially stimulate growth hormone production in pituitary gland cells. CJC-1295 is also modified by adding a drug affinity complex (DAC) component that may bind to plasma proteins. More specifically, the DAC component in CJC-1295 refers to the attachment of the N-epsilon-3-maleimidopropionamide derivative of lysine at the C terminus. By combining the tetrasubstituted amino acid sequence and the DAC component, CJC-1295 appears to exhibit improved pharmacokinetics while having a similar affinity to the GHRH receptors in the pituitary gland as native GHRH. More specifically, researchers comment that when the peptide was “selected for further pharmacokinetic evaluation, where it was found to be present in plasma beyond 72 h.”[1]
Ipamorelin is a synthetic pentapeptide that is considered to bind to another receptor found in pituitary gland cells, called growth hormone secretagogue receptor (GHS-R1a). These receptors are also found in the hypothalamus. Furthermore, GHS-R1a are also known as the ghrelin receptors, as ghrelin appears to be their main natural ligand. GHRP-2, or Growth Hormone Releasing Peptide 2, is a synthetic peptide composed of six amino acids. It also appears to bind to the ghrelin (GHS-R1a) receptors. By activating them, both Ipamorelin & GHRP-2 appear to stimulate the production of growth hormones in pituitary cells.
CJC-1295 & Ipamorelin & GHRP-2 and the GHRH Receptor
CJC-1295 & Ipamorelin & GHRP-2 may interact with various receptors found in the cells of the pituitary gland, such as the GHRH receptor. More specifically, the GHRH receptor is likely the primary target of CJC-1295.
When the peptide potentially interacts with the growth hormone-releasing hormone GHRH receptor, it appears to bind with specific binding sites on the receptor protein, which may lead to conformational changes in the receptor structure. This binding event may initiate a cascade of molecular events that appear to activate signal transduction pathways within the target cells.
The apparent conformational changes induced by CJC-1295 binding facilitate the potential activation of G-proteins, which are intracellular signaling proteins that may act as molecular switches. These G-proteins appear to be associated with the intracellular side of the GHRH receptor.[2] Activated G-proteins may stimulate the production of second messengers like cyclic adenosine monophosphate (cAMP) or inositol trisphosphate (IP3), which may serve as secondary signaling molecules, propagating the signal further into the cell.[3]
Second messengers such as cAMP may also activate protein kinases, which appear to be enzymes responsible for phosphorylating specific target proteins. Protein kinases may play a role in regulating various cellular processes. Activation of protein kinases might lead to the phosphorylation of transcription factors, which are proteins potentially involved in controlling gene expression. Once phosphorylated, these transcription factors may enter the nucleus and potentially modulate the transcription of specific genes associated with growth hormone synthesis and secretion. Ultimately, the molecular events triggered by CJC-1295 binding may result in the fusion of secretory vesicles containing growth hormone with the plasma membrane. This fusion enables the potential release of growth hormone outside the pituitary cells, where it may exert biological action.[4]
CJC-1295 & Ipamorelin & GHRP-2 and the GHS-R1a
The GHS-R1a receptors are found in the pituitary gland and the hypothalamus and appear to be the main targets of Ipamorelin & GHRP-2. To understand their molecular mechanisms of interaction with GHS-R1a, it’s important to delve into the structure and function of both the receptor and the peptides.
GHS-R1a is a G-protein coupled receptor (GPCR) that belongs to the class A rhodopsin-like family of receptors. It appears to play a potential role in regulating growth hormone release by pituitary cells into the extracellular environment.[5] Scientists comment, “since its discovery, hundreds of studies have shown the importance of this receptor and its endogenous ligand, ghrelin, in metabolism, neurotransmission, and behavior.” The receptor consists of seven transmembrane domains, an extracellular N-terminus, and an intracellular C-terminus. The extracellular N-terminus of GHS-R1a may have the potential for ligand binding to agonists such as Ipamorelin & GHRP-2, while the intracellular C-terminus interacts with G-proteins to initiate signaling pathways.[6]
Both Ipamorelin and GHRP-2 may act as agonists for GHS-R1a, meaning they potentially bind to the receptor and activate its signaling pathway. These peptides have a specific sequence of amino acids that allows them to potentially interact with GHS-R1a. The N-terminus of GHS-R1a contains binding sites that appear to recognize specific amino acid sequences in Ipamorelin and GHRP-2. When these peptides come into contact with the receptor, they appear to bind to these sites through non-covalent interactions such as hydrogen bonds, electrostatic interactions, and van der Waals forces. Upon Ipamorelin or GHRP-2 potentially binding to GHS-R1a, there appears to be a conformational change in the receptor. This change potentially leads to activating intracellular signaling pathways, primarily involving the G-proteins. GHS-R1a may interact with G-proteins, specifically the Gαq/11 subunit.[7]
Activation of Gαq/11 triggers the release of a signaling molecule called GTP (guanosine triphosphate) from GDP (guanosine diphosphate) bound to Gαq/11. The GTP-bound Gαq/11 subunit dissociates from the receptor and may activate downstream signaling events. Furthermore, the dissociation of Gαq/11 from GHS-R1a may initiate a series of intracellular signaling cascades.
One of the primary signaling pathways potentially activated by GHS-R1a involves the enzyme phospholipase C (PLC). Gαq/11 binds to PLC, which in turn may cleave a phospholipid called phosphatidylinositol 4,5-bisphosphate (PIP2) into two secondary messengers: inositol trisphosphate (IP3) and diacylglycerol (DAG).[8] IP3 appears to bind to receptors on the endoplasmic reticulum, causing the release of calcium ions (Ca2+) from intracellular stores. Furthermore, DAG may activate protein kinase C (PKC), phosphorylating downstream signaling molecules, further amplifying the signaling cascade. These signaling events potentially lead to the activation of protein kinases, transcription factors, and other regulatory proteins involved in the growth hormone release by pituitary cells into the extracellular environment.
Conclusion
In conclusion, CJC-1295 & Ipamorelin & GHRP-2 may interact with receptors in the central nervous system, particularly in the pituitary gland cells and hypothalamus. These peptides are hypothesized to have neuroendocrine actions, and their unique structures are directly linked to their secretagogue-like potential. CJC-1295 may bind to GHRH receptors in the pituitary gland, potentially leading to conformational changes and the activation of intracellular signaling pathways for growth hormone production. Ipamorelin and GHRP-2 may bind to GHS-R1a receptors, also known as ghrelin receptors which may initiate a cascade of molecular events involving G-proteins, second messengers, protein kinases, and transcription factors, potentially resulting in the release of growth hormone.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Jetté, L., Léger, R., Thibaudeau, K., Benquet, C., Robitaille, M., Pellerin, I., Paradis, V., van Wyk, P., Pham, K., & Bridon, D. P. (2005). Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology, 146(7), 3052–3058. https://doi.org/10.1210/en.2004-1286
- Martin, B., Lopez de Maturana, R., Brenneman, R., Walent, T., Mattson, M. P., & Maudsley, S. (2005). Class II G protein-coupled receptors and their ligands in neuronal function and protection. Neuromolecular medicine, 7(1-2), 3–36. https://doi.org/10.1385/nmm:7:1-2:003
- Newton, A. C., Bootman, M. D., & Scott, J. D. (2016). Second Messengers. Cold Spring Harbor perspectives in biology, 8(8), a005926. https://doi.org/10.1101/cshperspect.a005926
- Ionescu, M., & Frohman, L. A. (2006). Pulsatile secretion of growth hormone (GH) persists during continuous stimulation by CJC-1295, a long-acting GH-releasing hormone analog. The Journal of clinical endocrinology and metabolism, 91(12), 4792–4797. https://doi.org/10.1210/jc.2006-1702
- Albarrán-Zeckler, R. G., & Smith, R. G. (2013). The ghrelin receptors (GHS-R1a and GHS-R1b). Endocrine development, 25, 5–15. https://doi.org/10.1159/000346042
- Childs, M. D., & Luyt, L. G. (2020). A Decade’s Progress in the Development of Molecular Imaging Agents Targeting the Growth Hormone Secretagogue Receptor. Molecular imaging, 19, 1536012120952623. https://doi.org/10.1177/1536012120952623
- Yin, Y., Li, Y., & Zhang, W. (2014). The growth hormone secretagogue receptor: its intracellular signaling and regulation. International journal of molecular sciences, 15(3), 4837–4855. https://doi.org/10.3390/ijms15034837
- Bill, C. A., & Vines, C. M. (2020). Phospholipase C. Advances in experimental medicine and biology, 1131, 215–242. https://doi.org/10.1007/978-3-030-12457-1_9