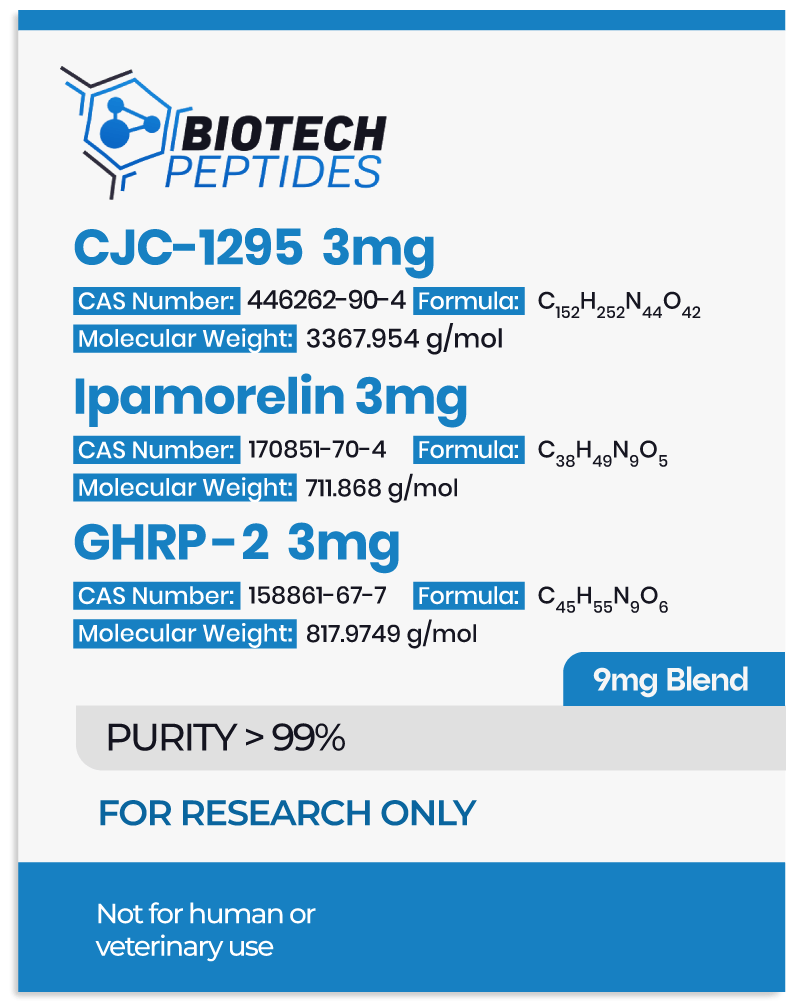
- CJC-1295 belongs to a class of molecules referred to as growth hormone-releasing hormone (GHRH) agonists.[1] It is made of the first 29 amino acids of the native GHRH hormone, representing the shortest functional sequence of GHRH. Further, CJC-1295 appears to have several modifications, including the replacement of four of the original amino acids in the GHRH 1-29 sequence, as well as the attachment of a rug affinity complex (DAC) component that may bind to plasma proteins. More specifically, the DAC component in CJC-1295 refers to the attachment of N-epsilon-3-maleimidopropionamide derivative at the C terminus. These modifications are posited to support the stability of the molecule and prolong its half-life.
- Ipamorelin, also referred to as NNC 26-0161, is a pentapeptide with the amino acid sequence Aib-His-D-2-Nal-D-Phe-Lys-NH2.[2] This molecule belongs to the family of growth hormone-releasing peptides (GHRPs), which were initially derived from the structure of endogenously occurring pentapeptides called metenkephalins. Ipamorelin appears to be highly selective towards triggering the release of growth hormone from the pituitary gland cells.
- GHRP-2 (growth hormone-releasing peptide-2) appears to belong to the GHRP class as well.[3] It is a hexapeptide developed from the structure of met-enkephalins. Yet, GHRP-2 appears to be less selective in its actions compared to Ipamorelin.
Mechanisms of Action
While it is believed that all three peptides may interact with the pituitary gland cells to stimulate growth hormone release, they may do so via different mechanisms:
- Interaction with the GHRH receptors: CJC-1295 appears to function primarily via the GHRH receptors found on pituitary gland cells. These receptors normally respond to the GHRH hormone, but CJC-1295 appears to induce similar activation. When CJC-1295 binds to this receptor on pituitary cells, it may activate intracellular proteins referred to as G-proteins. This might stimulate the production of second messengers such as cyclic adenosine monophosphate (cAMP) and inositol trisphosphate (IP₃). These small molecules are believed to serve as internal signals, potentially propagating the message deeper into the cell by relaying and amplifying the signal received at the cell surface. The increase in second messengers like cAMP is believed to activate enzymes called protein kinases. Once phosphorylated, these transcription factors may enter the cell nucleus and potentially modulate the transcription of genes involved in the synthesis and secretion of growth hormone.
- Interactions with ghrelin receptors: Ipamorelin and GHRP-2 appear to interact with a different subtype of pituitary receptors, called “ghrelin receptors” or also “growth hormone secretagogue receptor 1a” (GHS-R1a). These receptors normally respond to the hunger hormone ghrelin. GHRPs like GHRP-2 and Ipamorelin are also thought to activate these receptors and are consequently also referred to as growth hormone secretagogues. Once these peptides bind to the GHS-R1a, they appear to induce conformational changes that lead to the production of transcription factors. The latter may enter the pituitary cell nucleus and modulate the transcription of genes involved in the production and release of growth hormone.
Scientific and Research Studies
CJC-1295 & Ipamorelin & GHRP-2 Blend and Growth Hormone Signaling
All three of these peptides are believed to interact with the pituitary gland cells, which may potentially stimulate the release of growth hormone. The production of growth hormone may interact with receptors on the liver, muscle cells, and other cells, potentially initiating a series of intracellular signaling events such as activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) pathway.[4]
Following activation, STAT proteins may translocate to the nucleus and bind to specific DNA sequences referred to as response elements, which might facilitate the transcription of genes involved in the synthesis of insulin-like growth factor-1 (IGF-1). This is the main anabolic mediator of growth hormone, and CJC-1295 & Ipamorelin & GHRP-2 are expected to upregulate the synthesis of both the growth hormone and IGF-1 in experimental models. Unfortunately, no studies have investigated their potential as a blend, but several trials have experimented with each of the peptides individually and report the following observations:
- Experimental studies suggest that CJC-1295 may increase the synthesis of growth hormone by approximately 2- to 10-fold compared to placebo models.[5] Peak levels of growth hormone are reported to occur around two hours after introducing CJC-1295, and this elevated activity might persist for up to six days. Consequently, CJC-1295 may potentially contribute to average levels of IGF-1 increasing by about 1.5- to 3-fold throughout approximately 9 to 11 days. Additionally, repeated exposure to CJC-1295 appears to maintain elevated IGF-1 levels above baseline for up to 28 days.
- In studies involving Ipamorelin, it has been observed that growth hormone levels may increase significantly, potentially reaching up to 80 milli-International Units per liter (mIU/l), which corresponds to approximately 26.6 nanograms per milliliter (ng/ml).[6] Compared to the growth hormone levels around 1.31 mIU/l (0.4 ng/ml) with placebo, this represents what seems to be a substantial elevation in growth hormone concentration.
- Research on GHRP-2 suggests that it might stimulate growth hormone production from anterior pituitary cells up to 181 times the baseline levels.[7] Furthermore, some studies report that IGF-1 levels may increase from an average of 100 micrograms per liter (mcg/l) at baseline to approximately 180 mcg/l following GHRP-2 exposition. Another group of researchers found that GHRP-2 appears to stimulate the pulsatile, rhythmic, and entropic secretion of growth hormone by more than threefold compared to GHRH.[8]
CJC-1295 & Ipamorelin & GHRP-2 Blend and Hunger Hormone Signaling
While CJC-1295 appears to interact with the GHRH receptors, which do not affect hunger hormone signaling, Ipamorelin & GHRP-2 interact with the GHSR1a receptors, which are also referred to as the ghrelin receptors as the hunger hormone ghrelin activates them. Activation of GHSR1a receptors may promote the production of hunger hormone-stimulating neuropeptides, such as neuropeptide Y (NPY) and agouti-related peptide (AgRP), while potentially suppressing the release of alpha-melanocyte-stimulating hormone (α-MSH), an appetite-suppressing hormone.
Some studies have suggested that laboratory models exposed to Ipamorelin may experience a notable increase in hunger hormone signaling. This may lead to an increase in the size and weight of research models by approximately 15%, possibly due to a rise in adipose tissue relative to total mass.[9] Additionally, research indicates that models exposed to GHRP-2 may consume approximately 36% more food than control models, suggesting an increase in food intake relative to mass. Specifically, the energy intake per kilogram of mass in the GHRP-2 group was observed to be 136.0±13.0 kilojoules per kilogram, compared to 101.3±10.5 kilojoules per kilogram in the control group.[10]
CJC-1295 & Ipamorelin & GHRP-2 Blend in Bone Tissues
Some research suggests that Ipamorelin might support bone formation and possibly increase overall bone mass.[11,12] This hypothesis arises from observations implying that Ipamorelin might be associated with an apparent rise in bone mineral content (BMC), which refers to the total amount of minerals in bone tissue. In a particular study involving murine models, scientists examined the potential actions of Ipamorelin on bone mineral content. Researchers have proposed that Ipamorelin may lead to increases in the size, weight, and bone mineral content of experimental animals.
These changes might be measured using dual-energy X-ray absorptiometry (DXA). This non-invasive imaging technique assesses bone density by gauging how bones and soft tissues absorb X-rays. The researchers also commented, “that the increases in cortical and total BMC were due to an increased growth of the bones with increased bone dimensions, whereas the volumetric BMD was unchanged.” Therefore, Ipamorelin appears to mediate this potential by apparently upregulating anabolic growth signals like growth hormone and IGF-1. The addition of CJC-1295 and GHRP-2 may further upregulate this potential, although research is lacking.
CJC-1295 & Ipamorelin & GHRP-2 Blend in Muscle Cell Hypertrophy
The potential upregulation of growth hormone and IGF-1 levels by peptides like CJC-1295 & Ipamorelin & GHRP-2 is also expected to lead to increased anabolic signaling with muscular tissue, which is associated with muscle cell hypertrophy (increase in cell size). For example, preliminary research with CJC-1295 analogs suggests that the peptide may induce a notable increase in muscle tissue hypertrophy, leading to an average net gain of lean mass of about 2.77 pounds within 16 weeks.[13]
Researchers have also posited that “GHRP-2 enhanced muscle protein deposition mainly by
up-regulating the protein synthesis pathways” when conducting research in yaks. The study suggested that GHRP-2 may help to overcome endogenous growth limitations that occur in yaks because of food deprivation, adverse environmental conditions, and disease. Furthermore, GHRP-2 may have indicated potential contributions to reduction in atrophy of muscular tissue through repression of muscle cell-specific enzymes called E3 ubiquitin ligases—such as atrogin-1 and muscle ring finger protein-1 (MuRF1), which are believed to upregulate muscle cell degradation pathways. Specifically, these enzymes are thought to tag proteins for degradation via the ubiquitin-proteasome pathway, a cellular system responsible for breaking down proteins.
It is also theoretically possible that Ipamorelin may also help reduce the loss of muscle cells in catabolic experimental models. This potential might occur by increasing the IGF-1 within muscular tissue. For instance, studies suggest that Ipamorelin may decrease muscular tissue loss in research models exposed to corticosteroids, which are believed to induce the wasting of muscular tissues.[15] The mechanisms underlying this potential action might involve the suppression of atrogin-1 and MuRF1, mediated by IGF-1. By possibly downregulating these ligases, IGF-1 may reduce muscle cell protein degradation and assist in preserving muscle cells.[16]
CJC-1295 & Ipamorelin & GHRP-2 Blend Synergy
Given that CJC-1295 may operate through a different biological pathway than Ipamorelin and GHRP-2, which are synthetic growth hormone secretagogues, some researchers hypothesize that combining these compounds may produce a synergistic action on somatotroph cells and induce an even greater growth hormone synthesis. For example, some scientists point to studies that have investigated potential synergism between similar compounds.
For example, one study examined a CJC-1295 analog and GHRP-2, reporting that each peptide individually may have led to a 20-fold and 47-fold increase, respectively, in the pulsatile secretion of growth hormone from anterior pituitary somatotroph cells.[17] However, when both compounds were applied simultaneously, the increase in growth hormone secretion may have reached 54-fold, suggesting a possible synergistic interaction. More research is needed to investigate the potential synergy between CJC-1295 and GHRP-2 and how it may be affected by the addition of Ipamorelin.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- Jetté L, Léger R, Thibaudeau K, Benquet C, Robitaille M, Pellerin I, Paradis V, van Wyk P, Pham K, Bridon DP. Human growth hormone-releasing factor (hGRF)1-29-albumin bioconjugates activate the GRF receptor on the anterior pituitary in rats: identification of CJC-1295 as a long-lasting GRF analog. Endocrinology. 2005 Jul;146(7):3052-8. doi: 10.1210/en.2004-1286. Epub 2005 Apr 7. PMID: 15817669.
- Raun K, Hansen BS, Johansen NL, Thøgersen H, Madsen K, Ankersen M, Andersen PH. Ipamorelin, the first selective growth hormone secretagogue. Eur J Endocrinol. 1998 Nov;139(5):552-61. doi: 10.1530/eje.0.1390552. PMID: 9849822.
- Berlanga-Acosta J, Abreu-Cruz A, Herrera DGB, Mendoza-Marí Y, Rodríguez-Ulloa A, García-Ojalvo A, Falcón-Cama V, Hernández-Bernal F, Beichen Q, Guillén-Nieto G. Synthetic Growth Hormone-Releasing Peptides (GHRPs): A Historical Appraisal of the Evidences Supporting Their Cytoprotective Effects. Clin Med Insights Cardiol. 2017 Mar 2;11:1179546817694558. doi: 10.1177/1179546817694558. PMID: 28469491; PMCID: PMC5392015.
- Himpe E, Kooijman R. Insulin-like growth factor-I receptor signal transduction and the Janus Kinase/Signal Transducer and Activator of Transcription (JAK-STAT) pathway. Biofactors. 2009 Jan-Feb;35(1):76-81. doi: 10.1002/biof.20. PMID: 19319849.
- Teichman SL, Neale A, Lawrence B, Gagnon C, Castaigne JP, Frohman LA. Prolonged stimulation of growth hormone (GH) and insulin-like growth factor I secretion by CJC-1295, a long-acting analog of GH-releasing hormone, in healthy adults. J Clin Endocrinol Metab. 2006 Mar;91(3):799-805. doi: 10.1210/jc.2005-1536. Epub 2005 Dec 13. PMID: 16352683.
- Gobburu, J. V., Agersø, H., Jusko, W. J., & Ynddal, L. (1999). Pharmacokinetic-pharmacodynamic modeling of ipamorelin, a growth hormone releasing peptide, in human volunteers. Pharmaceutical research, 16(9), 1412–1416. https://doi.org/10.1023/a:1018955126402
- Veldhuis, J. D., Keenan, D. M., Bailey, J. N., Adeniji, A. M., Miles, J. M., & Bowers, C. Y. (2009). Novel relationships of age, visceral adiposity, insulin-like growth factor (IGF)-I and IGF binding protein concentrations to growth hormone (GH) releasing-hormone and GH releasing-peptide efficacies in men during experimental hypogonadal clamp. The Journal of clinical endocrinology and metabolism, 94(6), 2137–2143. https://doi.org/10.1210/jc.2009-0136
- Bowers, C. Y., Granda, R., Mohan, S., Kuipers, J., Baylink, D., & Veldhuis, J. D. (2004). Sustained elevation of pulsatile growth hormone (GH) secretion and insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), and IGFBP-5 concentrations during 30-day continuous subcutaneous infusion of GH-releasing peptide-2 in older men and women. The Journal of clinical endocrinology and metabolism, 89(5), 2290–2300. https://doi.org/10.1210/jc.2003-031799
- Lall, S., Tung, L. Y., Ohlsson, C., Jansson, J. O., & Dickson, S. L. (2001). Growth hormone (GH)-independent stimulation of adiposity by GH secretagogues. Biochemical and biophysical research communications, 280(1), 132–138. https://doi.org/10.1006/bbrc.2000.4065
- Laferrère, Blandine, et al. “Growth hormone-releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men.” The Journal of Clinical Endocrinology and Metabolism vol. 90,2 (2005): 611-4.
- Johansen PB, Nowak J, Skjaerbaek C, Flyvbjerg A, Andreassen TT, Wilken M, Orskov H. Ipamorelin, a new growth-hormone-releasing peptide, induces longitudinal bone growth in rats. Growth Horm IGF Res. 1999 Apr;9(2):106-13. doi: 10.1054/ghir.1999.9998. PMID: 10373343.
- Svensson J, Lall S, Dickson SL, Bengtsson BA, Rømer J, Ahnfelt-Rønne I, Ohlsson C, Jansson JO. The GH secretagogues ipamorelin and GH-releasing peptide-6 increase bone mineral content in adult female rats. J Endocrinol. 2000 Jun;165(3):569-77. Doi: 10.1677/joe.0.1650569. PMID: 10828840.
- Khorram, O., Laughlin, G. A., & Yen, S. S. (1997). Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women. The Journal of clinical endocrinology and metabolism, 82(5), 1472–1479. https://doi.org/10.1210/jcem.82.5.3943
- Hu R, Wang Z, Peng Q, Zou H, Wang H, Yu X, Jing X, Wang Y, Cao B, Bao S, Zhang W, Zhao S, Ji H, Kong X, Niu Q. Effects of GHRP-2 and Cysteamine Administration on Growth Performance, Somatotropic Axis Hormone and Muscle Protein Deposition in Yaks (Bos grunniens) with Growth Retardation. PLoS One. 2016 Feb 19;11(2):e0149461. doi: 10.1371/journal.pone.0149461. PMID: 26894743; PMCID: PMC4760683.
- Andersen, N. B., Malmlöf, K., Johansen, P. B., Andreassen, T. T., Ørtoft, G., & Oxlund, H. (2001). The growth hormone secretagogue ipamorelin counteracts glucocorticoid-induced decrease in bone formation in adult rats. Growth hormone & IGF research: official journal of the Growth Hormone Research Society and the International IGF Research Society, 11(5), 266–272. https://doi.org/10.1054/ghir.2001.0239
- Sacheck, J. M., Ohtsuka, A., McLary, S. C., & Goldberg, A. L. (2004). IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. American journal of physiology. Endocrinology and metabolism, 287(4), E591–E601. https://doi.org/10.1152/ajpendo.00073.2004
- Sinha, D. K., Balasubramanian, A., Tatem, A. J., Rivera-Mirabal, J., Yu, J., Kovac, J., Pastuszak, A. W., & Lipshultz, L. I. (2020). Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Translational andrology and urology, 9(Suppl 2), S149–S159. https://doi.org/10.21037/tau.2019.11.30