Tesamorelin (5mg & 10mg)
$38.00 – $73.00
Tesameorelin peptides are Synthesized and Lyophilized in the USA.
Discount per Quantity
| Quantity | 5 - 9 | 10 + |
|---|---|---|
| Discount | 5% | 10% |
| Price | $36.10 – $69.35 | $34.20 – $65.70 |
FREE - USPS priority shipping
Tesamorelin Peptide
Tesamorelin is a chemically altered growth hormone-releasing hormone (GHRH) analog, that similar to the original is made of 44 amino acids. This peptide is a trans-3-hexanoic acid version of natural GHRH. The trans-3-hexanoic acid group is added to the N-terminus, while the C-terminus is amidated and acetylated. Tesamorelin appears to mediate the positive influence of GHRH and other GHRH analogs such as GRF (1-29), CJC-1295, and Sermorelin. The trans-3-hexanoic acid modification may increase its stability and half-life. Both Tesamorelin and CJC-1295 appear to maintain the physiological activity of GHRH, without disrupting the physiological rhythm of GH release.
Specifications
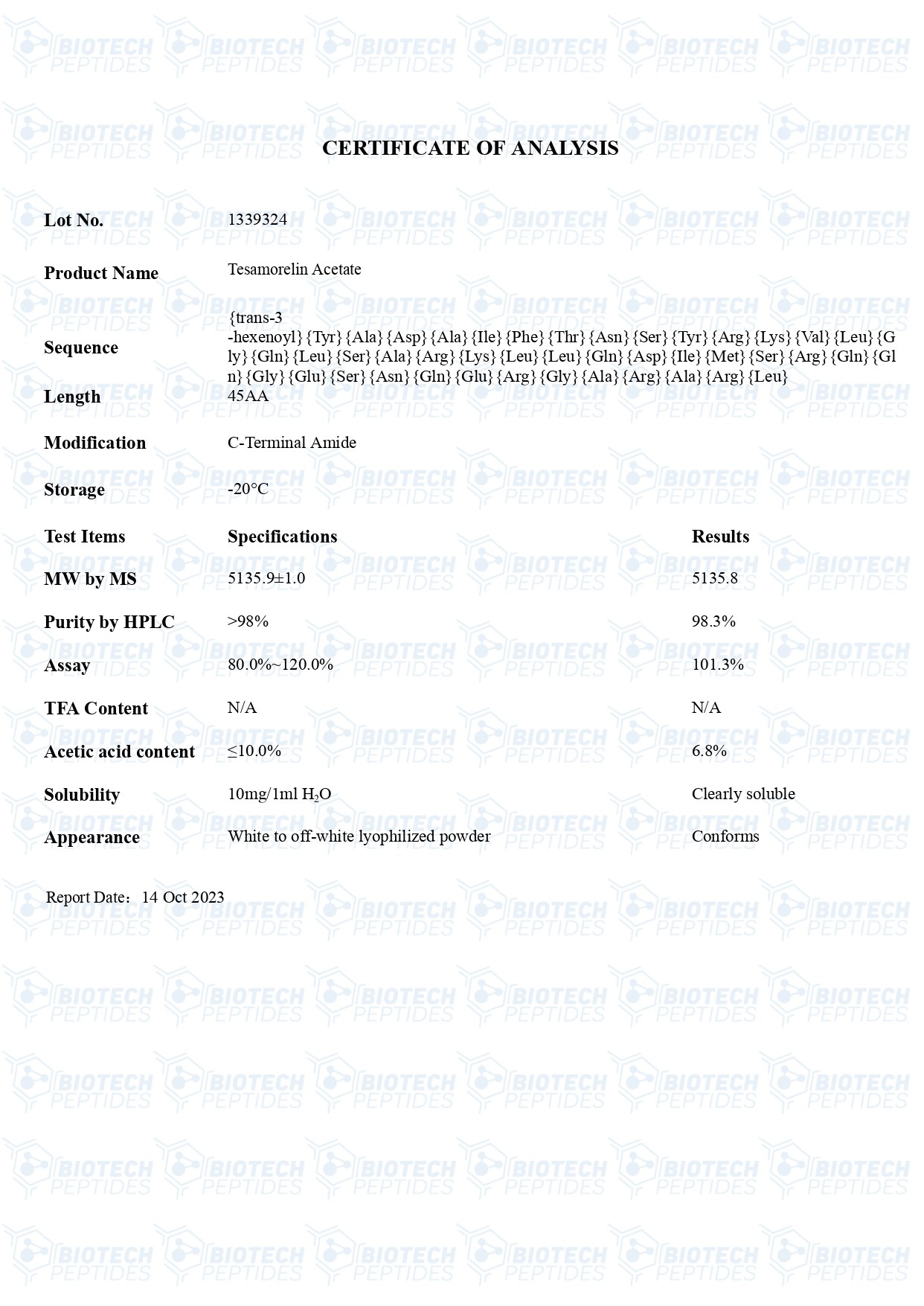
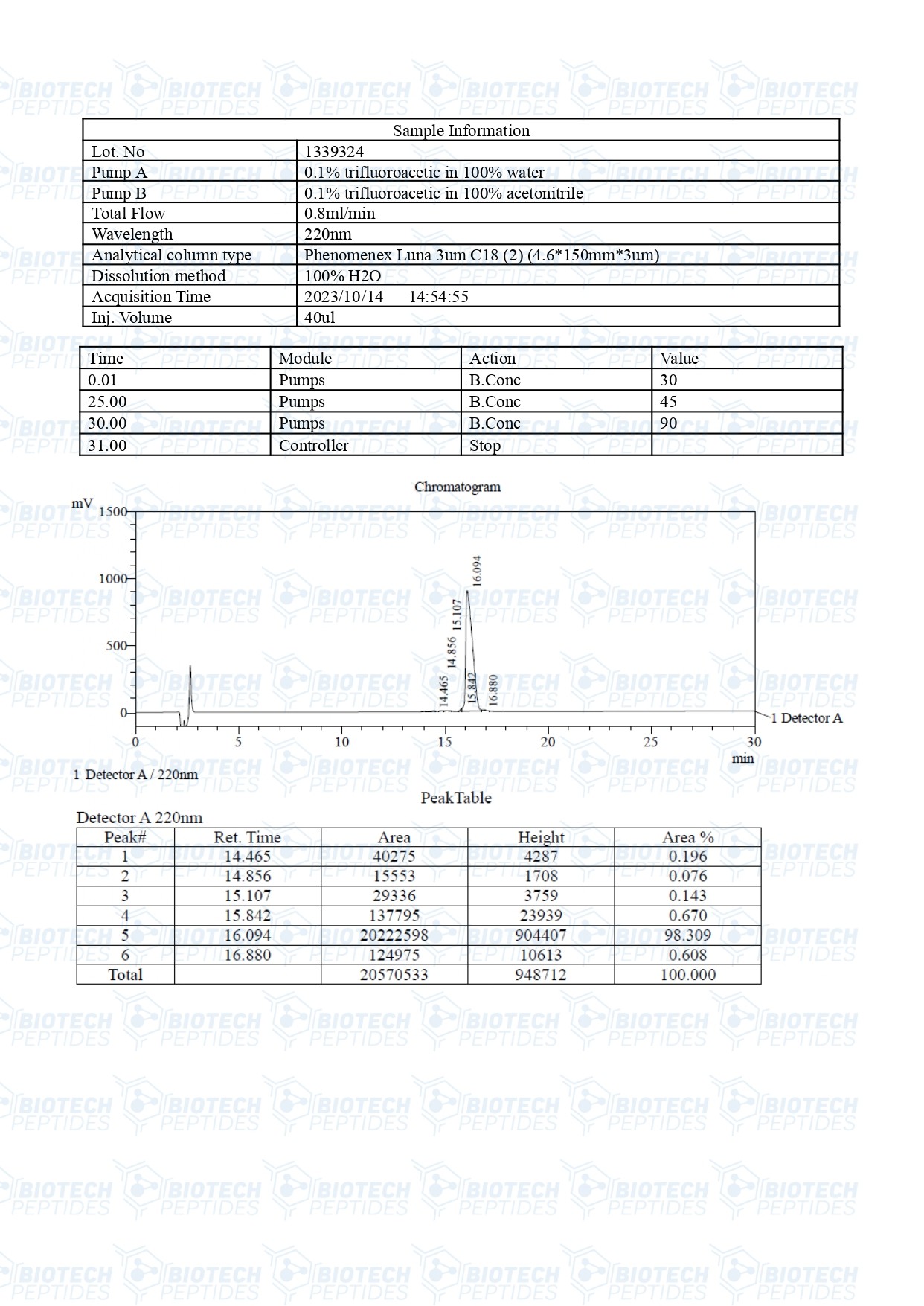
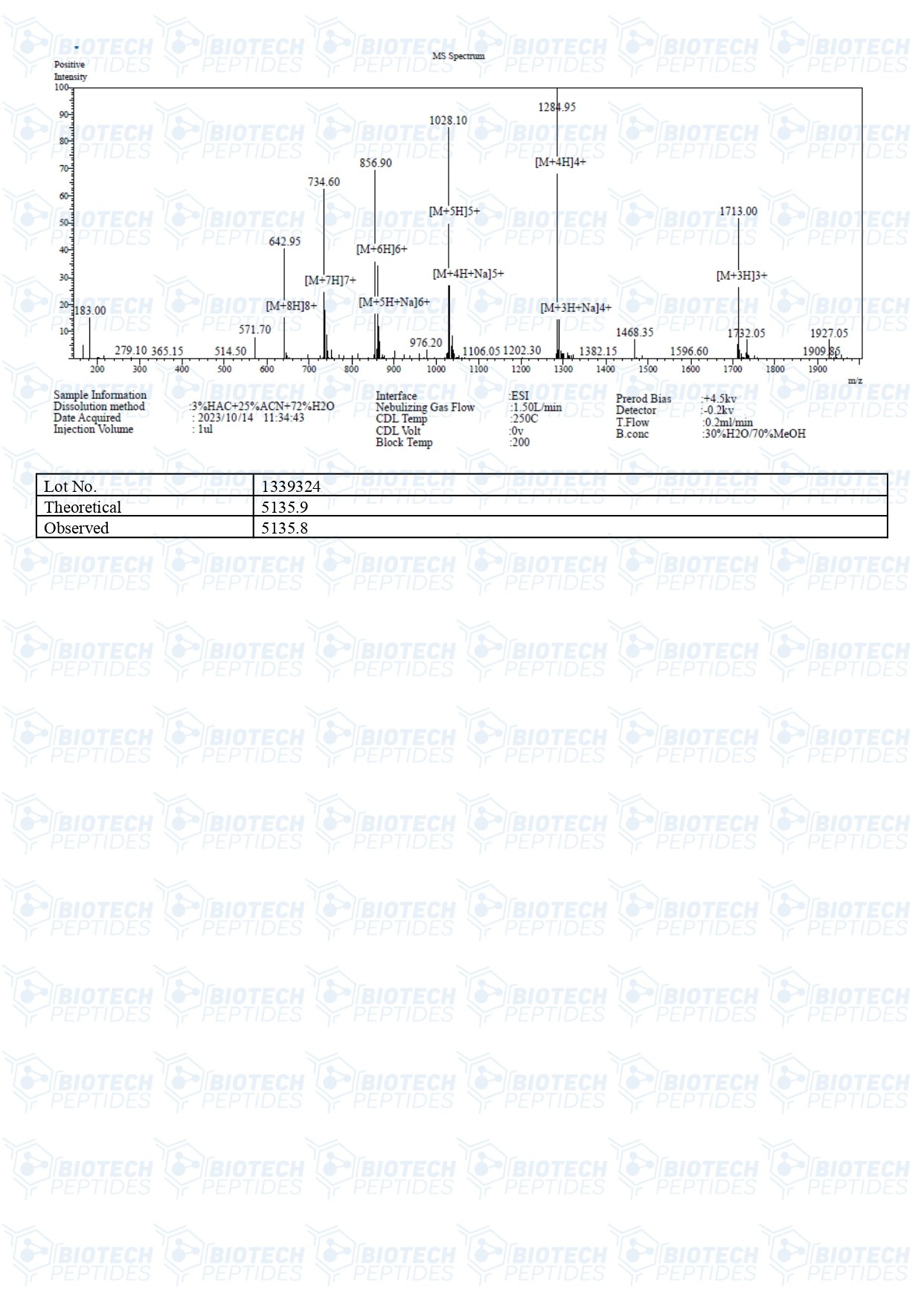
Molecular Formula: C221H366N72O67S
Molecular Weight: 5136 g/mol
Sequence: trans-hexenoyl-acid-Tyr-Ala-Asp-Ala-Ile-Phe-Thr-AsnSer-Tyr-Arg-Lys-Val-Leu-Gly-Gln-Leu-Ser-Ala-Arg-Lys-Leu-LeuGln-Asp-Ile-Met-Ser-Arg-GlnGln-Gly-Glu-Ser-Asn-Gln-Glu-Arg-Gly-Ala-Arg-Ala-Arg-Leu
Tesamorelin Research
Tesamorelin and the Pituitary Gland
Tesamorelin might potentially interact with the pituitary gland by possibly binding to GHRH receptors, which might initiate a sequence of molecular events. Researchers propose that it may cause structural alterations in the receptor, thereby activating intracellular signaling pathways.[1, 2] They have noted that this binding process is likely followed by a significant conformational change, involving the transmembrane helix 6 (TM6), which may open the intracellular face for G protein coupling. One potential pathway might involve the stimulation of cyclic adenosine monophosphate (cAMP) production within pituitary cells. This might be achieved through the activation of the enzyme adenylate cyclase, which may convert ATP (adenosine triphosphate) to cAMP. It is suggested that increased cAMP levels might activate protein kinase A (PKA), leading to protein phosphorylation and GHRH receptor activation by Tesamorelin. This cascade might stimulate the synthesis and secretion of growth hormone (hGH) from somatotrophs in the pituitary gland. Research indicates that Tesamorelin may induce up to a 69% rise in overall growth hormone levels, as measured by the 12-hour area under the curve (AUC), which quantifies the total hormone concentration over 12 hours. Additionally, there may be approximately a 55% increase in the average pulse area of growth hormone, which reflects the amount of hormone released during each pulse. Furthermore, levels of insulin-like growth factor 1 (IGF-1) apparently surged by 122%.[3]
Tesamorelin and Growth Hormone Deficiency, HIV
Highly active antiretroviral therapy (HAART) may trigger endocrine and metabolic disorders, including growth hormone (GH) deficiency. In cases of HIV infection, the pituitary gland function may be altered, inducing a general growth hormone deficiency in one-third of research models used to study the impacts of HAART.[4] Tesamorelin has been employed in research to measure its potential impact in supplementing growth hormone deficiency by inducing natural hormone production.
Tesamorelin and Lipodystrophy
Tesamorelin is principally researched within the context of HIV-associated lipodystrophy, which is considered to be caused by viral infection and possible adverse consequences of certain antiretroviral procedures. Lipodystrophy is characterized by an irregular distribution and storage of fat cells, which can often result in visceral obesity. Visceral obesity refers to the abnormal accumulation of fat around and within internal organs, and it has been tentatively linked to a range of metabolic disturbances. These disturbances may include insulin resistance, which impairs the organism’s ability to regulate blood sugar levels; elevated levels of low-density lipoprotein (LDL) cholesterol, often referred to as "bad" cholesterol due to its association with increased risk of cardiovascular disease; and hyperuricemia, which is an excessive concentration of uric acid in the blood. Tesamorelin has been hypothesized to potentially mitigate these metabolic disturbances due to its speculated action on adiposity. The peptide appeared to reduce adiposity by up to 20% of the models examined in one study.[5] The researchers noted that “The odds of response of VAT ‹140 cm2 was 3.9 times greater for Tesamorelin-... [cases] than … the control.” Another experiment that lasted for over 52 weeks and involved over 800 research models suggested that the peptide may lead to a mean of -17.5% reduction in visceral adiposity. In addition there were apparent reductions in triglycerides by a mean of -48 mg/dl, cholesterol by a mean of -8 mg/dl, and non-high-density lipoprotein by a mean of -7 mg/dl.[6] Further research into Tesamorelin that has involved reviews of multiple experiments has suggested it may lead to an apparent reduction of up to -25% reduction in visceral fat among lipodystrophy models.[7]
Tesamorelin and Cholesterol Metabolism
Ectopic fat deposition, such as in visceral organs, epicardium, and liver, has been linkedto elevated inflammation, which may increase the risk of lipids and cholesterol imbalance. Tesamorelin studies posit that the peptide may reduce triglyceride, total cholesterol, and non-HDL-C.[8] The peptide may potentially decrease inflammatory response through the control of excess adiposity.[9] The researchers note that “[models exposed to] Tesamorelin with ≥8% reduction in VAT have significantly improved triglyceride levels, adiponectin levels, and preservation of glucose homeostasis over 52 weeks.”
Tesamorelin and Peripheral Nerve Damage
Peripheral nerve damage may potentially trigger debilitating motor and sensory challenges. Research in intervention of such damage is limited, as nerve cells present a challenge to regenerate. Studies suggest that growth hormone manipulation might improve peripheral nerve injury and increase both rate and extent of repair.[10] Tesamorelin is being actively researched in this area for its potential for inducing growth hormone release.
Tesamorelin and Neurodegenerative Issues
GHRH analogs, including Tesamorelin, have been researched for their potential to improve cognitive ability in dementia models. A randomized, double-blind, placebo-controlled study was conducted with a large cohort over a period of 20 weeks at The University of Washington School of Medicine. The study observed that Tesamorelin and other GHRH analogs may influence dementia by increasing gamma-aminobutyric acid (GABA) in the brain and decreasing myo-insoitol (MI).[11] These findings suggest greater avenues of potential for Tesamorelin research.
Tesamorelin and Muscle Anabolism
A research study was conducted to explore the potential of Tesamorelin on the structural quality of muscle tissues, using computed tomography (CT) scans.[12] The findings from this study indicated a possible association between Tesamorelin and improvements in muscle tissue density and volume. Specifically, the study observed that certain muscle groups, namely the rectus abdominis, psoas major, and paraspinal muscles, exhibited more pronounced changes which included either an increase in muscle density and volume or a reduction in intramuscular fat content. From a statistical standpoint, the differences in muscle density and size, as well as the decrease in fat content within these muscles, were significantly greater than those observed in a control group.
Tesamorelin and Liver Adiposity
Research has indicated that Tesamorelin might lower hepatic fat fraction (HFF). [13] The study observed a reduction in absolute hepatic fat by 4.7% among Tesamorelin-exposed models, while the control group exhibited no change. This represents a relative decrease in liver fat by 37%, which might suggest a potential benefit in reducing liver fat accumulation. Furthermore, 35% of the Tesamorelin group achieved a hepatic fat fraction below 5%, in contrast to just 4% in the control. In terms of liver tissue fibrosis, Tesamorelin appeared to slow its progression, with only 10.5% of the Tesamorelin group showing fibrosis progression, compared to 37.5% in the placebo. Nevertheless, Tesamorelin did not seem to significantly improve pre-existing fibrosis. The observed reduction in liver fat was correlated with improvements in fibrosis, hinting at a possible mechanistic connection between reduced liver fat and decreased fibrosis progression. Additionally, Tesamorelin appeared to exhibit anti-inflammatory properties, as indicated by reductions in c-reactive protein (CRP) levels. Despite these promising findings, Tesamorelin did not significantly impact liver enzymes, such as alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT), overall. However, it did reduce ALT levels in models of elevated baseline levels. Metabolic parameters, including fasting glucose and hemoglobin A1c, were not significantly altered, suggesting that Tesamorelin might have a neutral action on glucose regulation during the study period.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Spooner, L. M., & Olin, J. L. (2012). Tesamorelin: a growth hormone-releasing factor analogue for HIV-associated lipodystrophy. The Annals of pharmacotherapy, 46(2), 240–247. https://doi.org/10.1345/aph.1Q629
- Zhou, F., Zhang, H., Cong, Z., Zhao, L. H., Zhou, Q., Mao, C., Cheng, X., Shen, D. D., Cai, X., Ma, C., Wang, Y., Dai, A., Zhou, Y., Sun, W., Zhao, F., Zhao, S., Jiang, H., Jiang, Y., Yang, D., Eric Xu, H., … Wang, M. W. (2020). Structural basis for activation of the growth hormone-releasing hormone receptor. Nature communications, 11(1),
- Stanley TL, Chen CY, Branch KL, Makimura H, Grinspoon SK. Effects of a growth hormone-releasing hormone analog on endogenous GH pulsatility and insulin sensitivity in healthy men. J Clin Endocrinol Metab. 2011 Jan;96(1):150-8. doi: 10.1210/jc.2010-1587. Epub 2010 Oct 13. PMID: 20943777; PMCID: PMC3038486.
- Rochira, V., & Guaraldi, G. (2017). Growth hormone deficiency and human immunodeficiency virus. Best practice & research. Clinical endocrinology & metabolism, 31(1), 91–111. doi:10.1016/j.beem.2017.02.006.
- Mangili, A., Falutz, J., Mamputu, J. C., Stepanians, M., & Hayward, B. (2015). Predictors of Treatment Response to Tesamorelin, a Growth Hormone-Releasing Factor Analog, in HIV-Infected Patients with Excess Abdominal Fat. PloS one, 10(10), e0140358. doi:10.1371/journal.pone.0140358.
- Falutz J, Mamputu JC, Potvin D, Moyle G, Soulban G, Loughrey H, Marsolais C, Turner R, Grinspoon S. Effects of Tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: a pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab. 2010 Sep;95(9):4291-304. doi: 10.1210/jc.2010-0490. Epub 2010 Jun 16. PMID: 20554713.
- Sivakumar T, Mechanic O, Fehmie DA, Paul B. Growth hormone axis treatments for HIV-associated lipodystrophy: a systematic review of placebo-controlled trials. HIV Med. 2011 Sep;12(8):453-62. doi: 10.1111/j.1468-1293.2010.00906.x. Epub 2011 Jan 25. PMID: 21265979.
- Falutz, J., Allas, S., Blot, K., Potvin, D., Kotler, D., Somero, M., Berger, D., Brown, S., Richmond, G., Fessel, J., Turner, R., & Grinspoon, S. (2007). Metabolic effects of a growth hormone-releasing factor in patients with HIV. The New England journal of medicine, 357(23), 2359–2370. doi:10.1056/NEJMoa072375.
- Stanley, T. L., Falutz, J., Marsolais, C., Morin, J., Soulban, G., Mamputu, J. C., Assaad, H., Turner, R., & Grinspoon, S. K. (2012). Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving Tesamorelin. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 54(11), 1642–1651. doi:10.1093/cid/cis251.
- Tuffaha, S. H., Singh, P., Budihardjo, J. D., Means, K. R., Higgins, J. P., Shores, J. T., Salvatori, R., Höke, A., Lee, W. P., & Brandacher, G. (2016). Therapeutic augmentation of the growth hormone axis to improve outcomes following peripheral nerve injury. Expert opinion on therapeutic targets, 20(10), 1259–1265. doi:10.1080/14728222.2016.1188079.
- Friedman, S. D., Baker, L. D., Borson, S., Jensen, J. E., Barsness, S. M., Craft, S., Merriam, G. R., Otto, R. K., Novotny, E. J., & Vitiello, M. V. (2013). Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA neurology, 70(7), 883–890. doi:10.1001/jamaneurol.2013.1425.
- Adrian S, Scherzinger A, Sanyal A, Lake JE, Falutz J, Dubé MP, Stanley T, Grinspoon S, Mamputu JC, Marsolais C, Brown TT, Erlandson KM. The Growth Hormone Releasing Hormone Analogue, Tesamorelin, Decreases Muscle Fat and Increases Muscle Area in Adults with HIV. J Frailty Aging. 2019;8(3):154-159. doi: 10.14283/jfa.2018.45. PMID: 31237318; PMCID: PMC6766405.
- Stanley, T. L., Fourman, L. T., Feldpausch, M. N., Purdy, J., Zheng, I., Pan, C. S., Aepfelbacher, J., Buckless, C., Tsao, A., Kellogg, A., Branch, K., Lee, H., Liu, C. Y., Corey, K. E., Chung, R. T., Torriani, M., Kleiner, D. E., Hadigan, C. M., & Grinspoon, S. K. (2019). Effects of Tesamorelin on non-alcoholic fatty liver disease in HIV: a randomised, double-blind, multicentre trial. The lancet. HIV, 6(12), e821–e830.