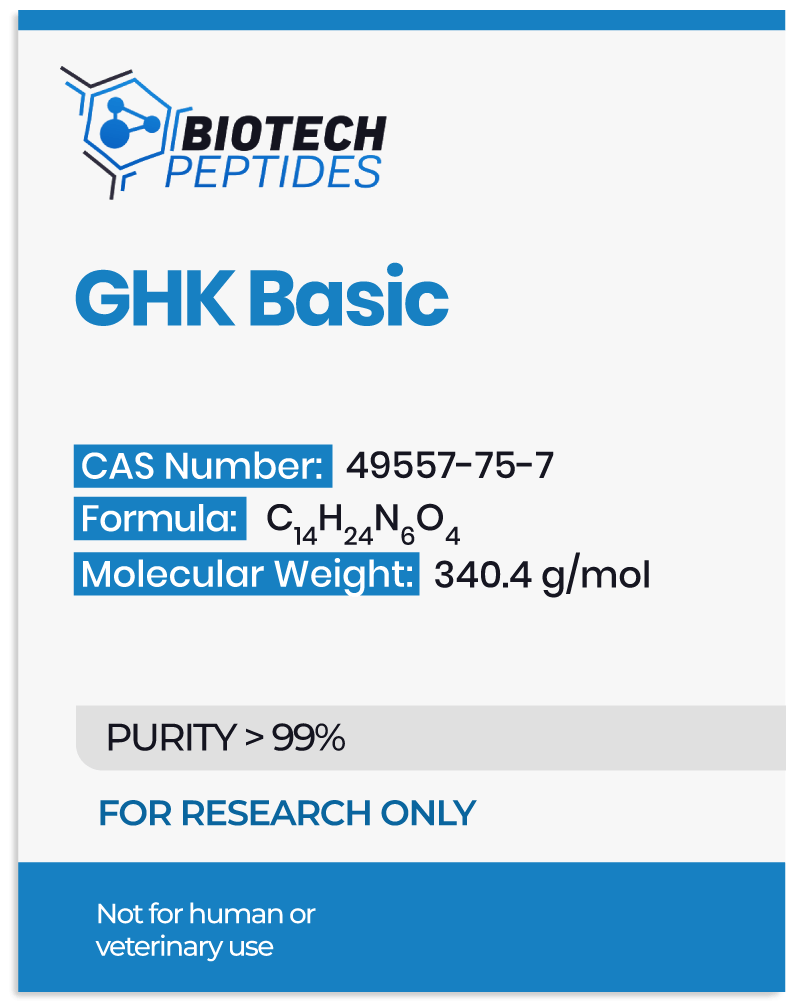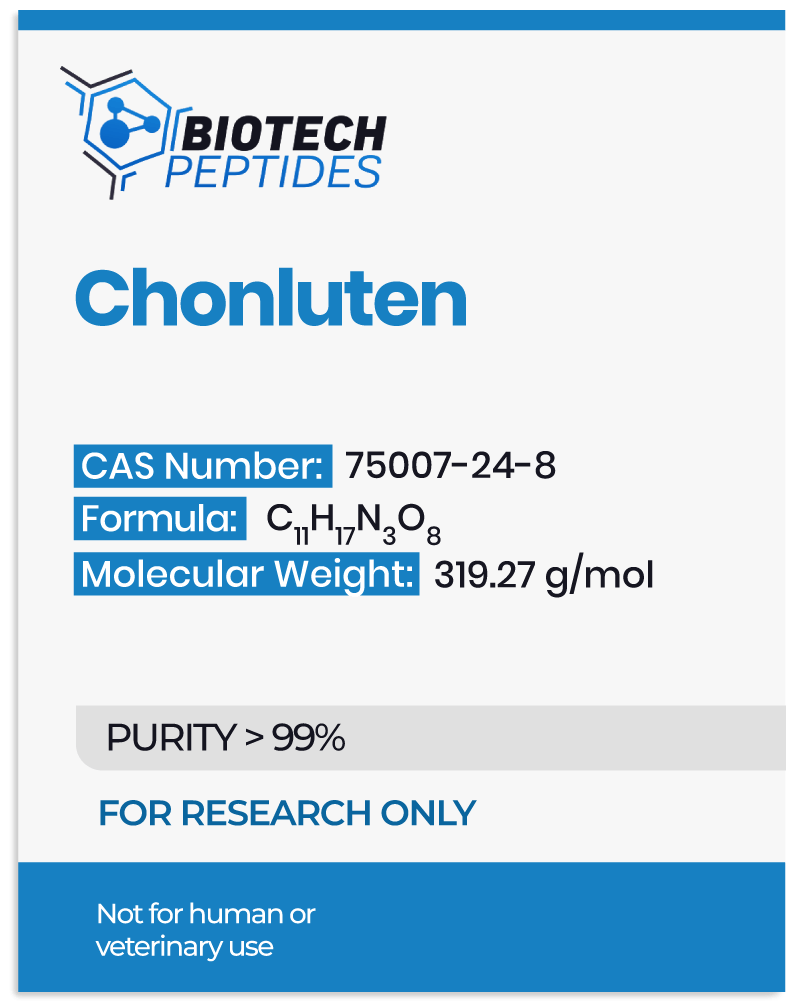
Pegylated MGF and Muscle Development
Pegylation may reduce immune response to a foreign body. In this case, Pegylated MGF peptide may increase the compound’s half-life in blood by reducing kidney clearance. Because MGF has a short lifespan in blood, PEG MGF peptide is an existing compound. Although MGF has been suggested to survive more extended periods in muscle tissues, it is considered to have a short life span.
Research
Pegylated MGF and Skeletal Muscles
According to research in mouse models with a muscular injury, the Mechano Growth Factor may protect the myoblasts by reducing the expression of certain inflammatory hormones and decreasing oxidative stress.[2] Research by Sun et al. suggests that MGF may regulate muscle inflammation and enhance macrophage and neutrophil recruitment to the injury site.[3]
A study by an international group of endocrinology researchers suggested that MGF activates the insulin-like growth factor 1 receptors in the same way as IGF-1.[4] Better energy homeostasis, enhanced lean body mass, and reduced cell aging may result from activating the IGF-1 receptor, suggesting that Pegylated MGF peptide may produce an action similar to to IGF-1. The product may be a net increase in lean body mass, increased fat metabolism, and activated muscle repair. Physical output in mice, according to research, may exhibit up to a 25% increase in mean muscle fiber size following MGF exposure.[5]
Pegylated MGF and Bone
Pegylated MGF has shown promise in bone repair rate in rabbits by reportedly increasing osteoblast proliferation during the course of scientific study in a laboratory. Osteoprogenitor cells may stimulate and secrete bone matrix, and participate in bone mineralization (bone tissue formation).
Pegylated MGF and the Heart
Research findings from the University of Illinois, Department of Bioengineering suggest that MGF may ameliorate apoptosis by cardiac muscle cells following hypoxia.[6] Pegylated MGF peptide may recruit cardiac stem cells to the injury site and might induce healing and regeneration following cardiac arrest.
Research has also suggested that localized MGF might improve cardiac function by reducing pathologic hypertrophy.[7] Scientists suggested improved hemodynamics and low cardiac remodeling rates in remodeling mice compared to mice without MGF exposure. A study by Carpenter et al. suggested that MGF in the disease condition of acute myocardial infarction might induce cardiomyocyte injury reduction by approximately 35%.
Pegylated MGF peptide appears to promote osteogenic differentiation and the expression of MMP-1 and MMP-2 in periodontal ligament samples. These factors may enable the repair of ligaments attached end to end. They may proffer surrogate extractions and implants following damage.
Pegylated MGF and Neuroprotection
A study reviewed by Alexander Walker, Editorial Assistant at BioMed Central, explored the long-term action of enhanced MGF levels in the central nervous system and brain.[8] The study suggested that high MGF levels may reduce the effects of age-dependent neuron degeneration. This study suggested that cognitive functions maintained peak performance over a prolonged duration. According to the editorial assistant, “MGF potency [may be] age-dependent”.
Mice models of ALS exposed to MGF exhibited better muscle weakness and decrease the loss of motor neurons. Dłużniewska et al. suggest that MGF expression may naturally take place in the brain following hypoxic injury and may be over-expressed in regions of the brain where neuron regeneration is highest.[9] Exogenous MGF might limit the impact of numerous neurological diseases by preventing neuron death in the spinal cord and brain despite the ongoing disease condition.
Pegylated MGF and Cartilage
Research suggests that MGF may improve chondrocyte functions—cells that regulate cartilage deposition. Mice model research suggest that MGF may increase chondrocyte migration from bone to cartilage where they impact. Research in this area is ongoing.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Kandalla PK, Goldspink G, Butler-Browne G, Mouly V. Mechano Growth Factor E peptide (MGF-E), derived from an isoform of IGF-1, activates human muscle progenitor cells and induces an increase in their fusion potential at different ages. Mech Ageing Dev. 2011 Apr;132(4):154-62. doi: 10.1016/j.mad.2011.02.007. Epub 2011 Feb 25. PMID: 21354439.
- Zabłocka B, Goldspink PH, Goldspink G, Górecki DC. Mechano-Growth Factor: an important cog or a loose screw in the repair machinery? Front Endocrinol (Lausanne). 2012 Nov 1;3:131. doi: 10.3389/fendo.2012.00131. PMID: 23125840; PMCID: PMC3485521.
- Sun KT, Cheung KK, Au SWN, Yeung SS, Yeung EW. Overexpression of Mechano-Growth Factor Modulates Inflammatory Cytokine Expression and Macrophage Resolution in Skeletal Muscle Injury. Front Physiol. 2018 Jul 26;9:999. doi: 10.3389/fphys.2018.00999. PMID: 30140235; PMCID: PMC6094977.
- Janssen JA, Hofland LJ, Strasburger CJ, van den Dungen ES, Thevis M. Potency of Full-Length MGF to Induce Maximal Activation of the IGF-I R Is Similar to Recombinant Human IGF-I at High Equimolar Concentrations. PLoS One. 2016 Mar 18;11(3):e0150453. doi: 10.1371/journal.pone.0150453. PMID: 26991004; PMCID: PMC4798685.
- Goldspink G. Research on mechano growth factor: its potential for optimising physical training as well as misuse in doping. Br J Sports Med. 2005 Nov;39(11):787-8; discussion 787-8. doi: 10.1136/bjsm.2004.015826. PMID: 16244184; PMCID: PMC1725070.
- Carpenter V, Matthews K, Devlin G, Stuart S, Jensen J, Conaglen J, Jeanplong F, Goldspink P, Yang SY, Goldspink G, Bass J, McMahon C. Mechano-growth factor reduces loss of cardiac function in acute myocardial infarction. Heart Lung Circ. 2008 Feb;17(1):33-9. doi: 10.1016/j.hlc.2007.04.013. Epub 2007 Jun 19. PMID: 17581790.
- Peña JR, Pinney JR, Ayala P, Desai TA, Goldspink PH. Localized delivery of mechano-growth factor E-domain peptide via polymeric microstructures improves cardiac function following myocardial infarction. Biomaterials. 2015 Apr;46:26-34. doi: 10.1016/j.biomaterials.2014.12.050. Epub 2015 Jan 16. PMID: 25678113; PMCID: PMC4328136.
- Tang JJ, Podratz JL, Lange M, Scrable HJ, Jang MH, Windebank AJ. Mechano growth factor, a splice variant of IGF-1, promotes neurogenesis in the aging mouse brain. Mol Brain. 2017 Jul 7;10(1):23. doi: 10.1186/s13041-017-0304-0. PMID: 28683812; PMCID: PMC5501366.
- Dluzniewska J, Sarnowska A, Beresewicz M, Johnson I, Srai SK, Ramesh B, Goldspink G, Górecki DC, Zabłocka B. A strong neuroprotective effect of the autonomous C-terminal peptide of IGF-1 Ec (MGF) in brain ischemia. FASEB J. 2005 Nov;19(13):1896-8. doi: 10.1096/fj.05-3786fje. Epub 2005 Sep 6. PMID: 16144956.