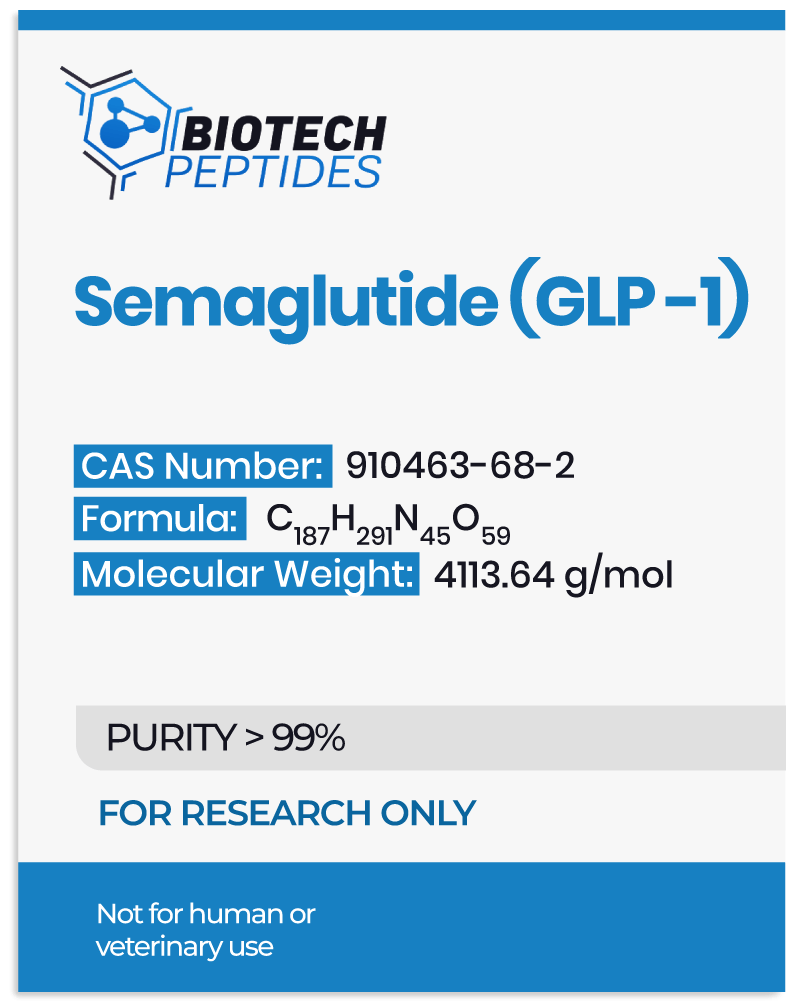
Semaglutide Research in Hormone Signaling
Semaglutide and Blood Sugar Control
Semaglutide has been hypothesized to act by activating the GLP-1 receptors in the pancreatic beta cells, stimulating insulin synthesis and release.[1][2] The stimulating effect on insulin synthesis is the primary mechanism via which Semaglutide may possibly lower both fasting and postprandial glucose levels. A meta-analysis of 26 RCTs suggests that Semaglutide may lower the fasting blood sugar levels and markers for long-term glucose control, such as HbA1c, in cases of type 2 diabetes.[3]
In addition to stimulating insulin secretion, Semaglutide may potentially reduce glucagon release and suppress hepatic gluconeogenesis.[4] These potential actions are supported by study findings in non-diabetic models, which lasted up to 12 weeks and reported over 38% reduction in blood sugar levels compared to a placebo after a carbohydrate-rich food delivery.[5] The researchers also suggested that Semaglutide may have slowed the speed of gastric emptying during the first hour after caloric intake compared to a placebo. The scientists suggested that this potential of Semaglutide may contribute to a gradual release of glucose and better glycemic control. Yet, the overall speed of gastric emptying over the entire 5-hour monitoring period after the meal appeared not affected.
Semaglutide has been hypothesized to reduce hyperglycemia without causing hypoglycemia. The risk of hypoglycemia is not considered to be higher when compared to a placebo as Semaglutide may possibly stimulate insulin secretion in a glucose-dependent manner.[6] In addition, the inhibition of glucagon release may not occur under hypoglycemic conditions.
Semaglutide and Weight
Semaglutide has been suggested to stimulate insulin secretion without leading to weight gain. Studies suggest that Semaglutide may reduce ad libitum energy intake, which may result in weight loss in the long term.[8] According to one study, Semaglutide reduced hunger hormone signaling to the brain, resulting in a reported 24% reduction in energy intake. Semaglutide may activate the GLP-1 receptors in the brain, which may play a major role in modulating appetite and reward-related behavior.[9] Furthermore, the potential of Semaglutide to slow down gastric emptying within the first hour of having a meal may also contribute to a reduced ad libitum energy intake.
Pancreatic Beta Cell Survival
Preliminary studies conducted in test animals suggest that Semaglutide may stimulate pancreatic beta cells’ survival and proliferation. These potential actions are considered to be of significant interest since cases of type 2 diabetes are often associated with pancreatic beta cell dysfunction and apoptosis in the long term.[10]
Animal research suggests that Semaglutide may help reverse the harmful changes of obesity and insulin resistance on pancreatic beta cells and stimulate their proliferation.[11] Researchers reveal that some studies also report that GLP-1 antagonists, such as Semaglutide, may protect pancreatic beta cells from apoptosis.[12] Several possible mechanisms are suggested in the protective potential of Semaglutide, and one of the most prominent is reducing the overload on the endoplasmic reticulum of the beta cells in diabetic conditions. GLP-1 receptor activation may also help stimulate autophagy, which prevents beta cell injury and death by protecting against inflammation and oxidative stress.
Semaglutide and Neuroprotection
Interestingly, Parkinson’s disease and type 2 diabetes are considered to share several genetic susceptibilities, such as single nucleotide polymorphisms in the growth factor signaling kinase gene Akt.[13] This has sparked interest in researching the potential of diabetes compounds for research studies on Parkinson’s disease. Currently, other GLP-1 receptor agonists, such as Exendin-4, have already been suggested to exhibit protective effects on Parkinson’s cases.[14] Another GLP-1 antagonist, Liraglutide, is under investigation for this hypothetical action.[16]
The research regarding the potential neuroprotective action of Semaglutide is still in its infancy, but many laboratory studies in animal models of PD suggest promising results.[15] The experiments report that Semaglutide may have neuroprotective characteristics and may increasethe survival of the dopaminergic neurons, the apoptosis of which is associated with the development of Parkinson’s.
In animals, Semaglutide appeared to have alleviated the chronic inflammatory responses in the brain, reduced lipid peroxidation, and increased the expression of growth factors that protect dopaminergic neurons in the substantia nigra and striatum.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- Dhillon S. Semaglutide: First Global Approval. Drugs. 2018 Feb;78(2):275-284. DOI: 10.1007/s40265-018-0871-0. PMID: 29363040.
- Hou Y, Ernst SA, Heidenreich K, Williams JA. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am J Physiol Gastrointest Liver Physiol. 2016 Jan 1;310(1):G26-33. doi: 10.1152/ajpgi.00293.2015. Epub 2015 Nov 5. PMID: 26542397; PMCID: PMC4698438.
- Zaazouee MS, Hamdallah A, Helmy SK, Hasabo EA, Sayed AK, Gbreel MI, Elmegeed AA, Aladwan H, Elshanbary AA, Abdel-Aziz W, Elshahawy IM, Rabie S, Elkady S, Ali AS, Ragab KM, Nourelden AZ. Semaglutide for the treatment of type 2 Diabetes Mellitus: A systematic review and network meta-analysis of safety and efficacy outcomes. Diabetes Metab Syndr. 2022 Jun;16(6):102511. DOI: 10.1016/j.dsx.2022.102511. Epub 2022 May 20. PMID: 35623229.
- Mahapatra MK, Karuppasamy M, Sahoo BM. Semaglutide, a glucagon like peptide-1 receptor agonist with cardiovascular benefits for management of type 2 diabetes. Rev Endocr Metab Disord. 2022 Jun;23(3):521-539. DOI: 10.1007/s11154-021-09699-1. Epub 2022 Jan 7. PMID: 34993760; PMCID: PMC8736331.
- Hjerpsted JB, Flint A, Brooks A, Axelsen MB, Kvist T, Blundell J. Semaglutide improves postprandial glucose and lipid metabolism, and delays first-hour gastric emptying in subjects with obesity. Diabetes Obes Metab. 2018 Mar;20(3):610-619. DOI: 10.1111/dom.13120. Epub 2017 Oct 27. PMID: 28941314; PMCID: PMC5836914.
- Smits MM, Van Raalte DH. Safety of Semaglutide. Front Endocrinol (Lausanne). 2021 Jul 7;12:645563. doi: 10.3389/fendo.2021.645563. Erratum in: Front Endocrinol (Lausanne). 2021 Nov 10;12:786732. PMID: 34305810; PMCID: PMC8294388.
- Mares AC, Chatterjee S, Mukherjee D. Semaglutide for weight loss and cardiometabolic risk reduction in overweight/obesity. Curr Opin Cardiol. 2022 Jul 1;37(4):350-355. DOI: 10.1097/HCO.0000000000000955. Epub 2022 Feb 16. PMID: 35175229.
- Blundell J, Finlayson G, Axelsen M, Flint A, Gibbons C, Kvist T, Hjerpsted JB. Effects of once-weekly Semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017 Sep;19(9):1242-1251. DOI: 10.1111/dom.12932. Epub 2017 May 5. PMID: 28266779; PMCID: PMC5573908.
- van Bloemendaal L, IJzerman RG, Ten Kulve JS, Barkhof F, Konrad RJ, Drent ML, Veltman DJ, Diamant M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014 Dec;63(12):4186-96. DOI: 10.2337/db14-0849. Epub 2014 Jul 28. PMID: 25071023.
- Tomita T. Apoptosis in pancreatic β-islet cells in Type 2 diabetes. Bosn J Basic Med Sci. 2016 Aug 2;16(3):162-79. DOI: 10.17305/bjbms.2016.919. Epub 2016 May 22. PMID: 27209071; PMCID: PMC4978108.
- Marinho TS, Martins FF, Cardoso LEM, Aguila MB, Mandarim-de-Lacerda CA. Pancreatic islet cells disarray, apoptosis, and proliferation in obese mice. The role of Semaglutide treatment. Biochimie. 2022 Feb;193:126-136. doi: 10.1016/j.biochi.2021.10.017. Epub 2021 Nov 4. PMID: 34742857.
- Costes S, Bertrand G, Ravier MA. Mechanisms of Beta-Cell Apoptosis in Type 2 Diabetes-Prone Situations and Potential Protection by GLP-1-Based Therapies. Int J Mol Sci. 2021 May 18;22(10):5303. doi: 10.3390/ijms22105303. PMID: 34069914; PMCID: PMC8157542.
- Xiromerisiou G, Hadjigeorgiou GM, Papadimitriou A, Katsarogiannis E, Gourbali V, Singleton AB. Association between AKT1 gene and Parkinson’s disease: a protective haplotype. Neurosci Lett. 2008 May 9;436(2):232-4. doi: 10.1016/j.neulet.2008.03.026. Epub 2008 Mar 15. PMID: 18395980; PMCID: PMC8958471.
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017 Oct 7;390(10103):1664-1675. DOI: 10.1016/S0140-6736(17)31585-4. Epub 2017 Aug 3. PMID: 28781108; PMCID: PMC5831666.
- Zhang L, Zhang L, Li L, Hölscher C. Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease. J Parkinsons Dis. 2019;9(1):157-171. DOI: 10.3233/JPD-181503. PMID: 30741689.
- Clinical trial identifier NCT02953665