Thymosin Alpha 1: General Research and Scientific Observations
Regarding the isolation, purification, and preparation of Thymosin Alpha 1, it was initially isolated from the calf thymus in 1977. However, a solid-phase synthesis technique in which molecules are covalently bound to a solid material is predominantly used for its commercial production. With the arrival of molecular expression methods, Ta1 is also being produced through prokaryotic and eukaryotic expression methodologies.
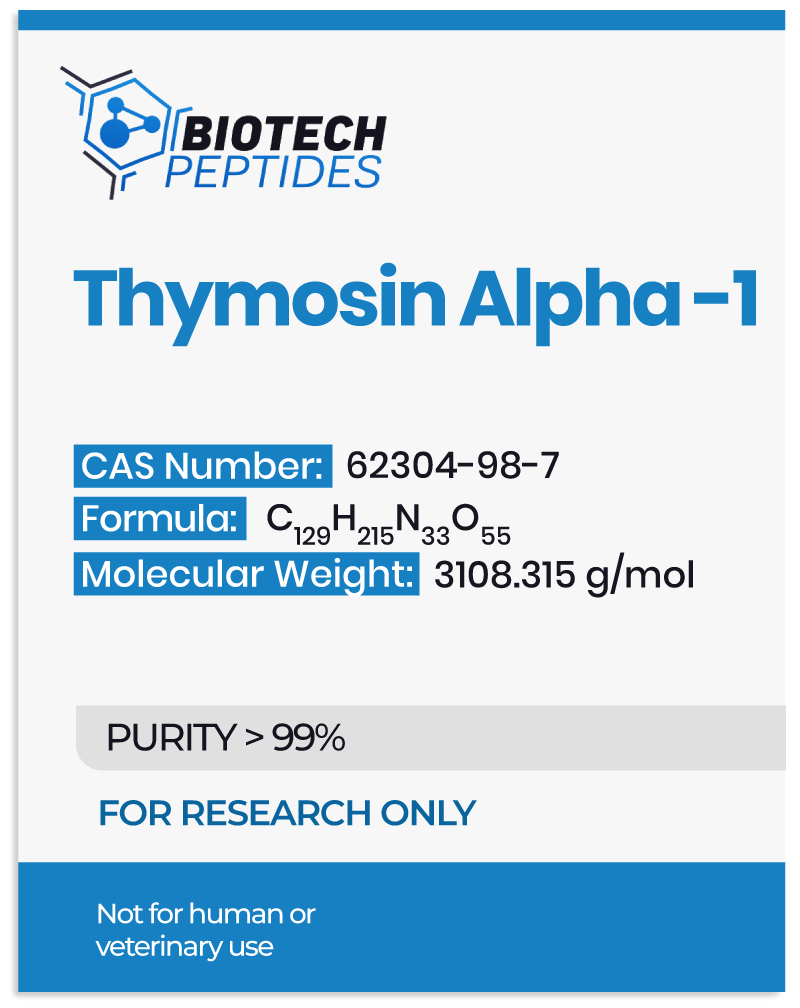
Thymosin Alpha 1 Overview
The synthetic analog of Thymosin Alpha 1, namely Thymalfasin, is primarily studied in research models of hepatitis B and C infection. It has the following amino acid sequence:
Ac–S–D–A–A–V–Asp–T–S–S–E–I–T–T–K–D–L–K–E–K–K–E–V–V–E–E–A–E–N–OH.
As described above and shown in the sequence, the peptide is N-terminally acetylated, has a net positive charge, and lacks stable conformation.
Several research institutions and teams have aimed to explore the breadth of the peptide’s potential. However, the mechanisms by which this peptide exerts its effects are an active area of investigation. Scientists believe that Thymosin Alpha 1’s potential in overcoming infections, cancer, immunodeficiency, and cell aging, may be through pleiotropic action. A recent study suggested that Thymosin Alpha 1 interactions with an oligosaccharide binding protein, Galectin -1 (Gal-1), are necessary to activate its potential [1].
The immunostimulatory effects of Thymosin Alpha-1 peptide are supposed to be mediated through Toll-like receptors (TLRs). It has been observed that Thymosin Alpha 1, through its binding with TLR3/4/9, appears to stimulate interferon regulatory factor (IRF3) and NF-kB signaling pathways. The ultimate effect is reflected in the immune-enhancing potential for both innate and adaptive immune system machinery. The immunomodulation against viral infections also appears to be mediated through activating T cells, B cells, macrophages, and Natural Killer cells [2].
The molecular mechanisms underlying the potential of Ta1 in controlling cellular proliferation through inducing apoptosis of cancerous cells are also being evaluated. Scientific studies suggest that the hypothetical anti-cancer action of Thymosin Alpha 1 may be mediated through the PI3K/Akt/mTOR signaling pathway. Furthermore, PTEN appears to mediate apoptosis through Thymosin Alpha 1 [3]. The specificity of this is yet to be deciphered through further studies.
It is clear from the above-described studies that researchers general assert that Thymosin Alpha 1 appears to exert action through differential molecular and cellular mechanisms that should be investigated in further detail.
Thymosin Alpha 1 Peptide Research
Scientific studies have provided ample data to support its hypothetical consequence within the context of various ailments.
The primary objectives of several ongoing research studies are to evaluate this peptide’s potential individually or in combination with other compounds. Mainly, its action as a sole agent in the research of immune reconstitution disorders, hepatocellular carcinoma, osteoporotic pain, rheumatic heart disease, sepsis, and viral infections, is an active area of scientific investigation. The peptide, in combination with other compounds, is being evaluated for advanced refractory solid tumors, esophageal cancer, rectal carcinoma, malignant melanoma, and non-small cell lung cancer (NSCLC).
Besides prospective well-designed studies, several retrospective evaluations hypothesize the relevance of this peptide in various avenues of scientific research. Particularly in sepsis, organ dysfunction is a major focus. A study compared the benefits of Thymosin Alpha 1 with a placebo. The outcome revealed that Ta1 reduces organ damage among research models of sepsis [4].
Another study evaluated the potential of Thymosin Alpha 1 on long-term survival in margin-free (R0)-resected stage IA–IIIA with non-small cell lung cancer (NSCLC) models. Findings suggested that Thymosin Alpha 1 exposure appeared to have improved disease-free survival after R0 resection. The period of the study spanned 24 months [5].
The United States Patent Application Publication US 2010/0004174 A1 published in the year 2010 claimed that Thymosin Alpha 1 may exhibit potential benefits in cases of multiple sclerosis, inflammatory bowel disease, Crohn’s disease/ulcerative colitis.
Conclusion
Thymosin Alpha 1 is an endogenous peptide, whose synthetic analog, Thymalfasin, is widely researched as a prospective agent in various ailments. Most prominent theorized actions of Thymosin Alpha 1 are speculated to encompass tumor growth and cancer suppression, boosting immune cell functions, mitigating bacterial, viral, and fungal infections, acting as an anti-inflammatory agent, and augmenting vaccine activity. The most plausible explanation for its diverse potential is that Thymosin Alpha 1 may mediate homeostasis within different organs and systems. However, diverse mechanisms operate to control different diseases. Though a broader profile, this peptide molecular mechanism still needs to be elucidated for each proposed indication.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References
- Matteucci C, Nepravishta R, Argaw-Denboba A, Mandaliti W, Giovinazzo A, Petrone V, Balestrieri E, Sinibaldi-Vallebona P, Pica F, Paci M, Garaci E. Thymosin alpha1 interacts with Galectin-1 modulating the beta-galactosides affinity and inducing alteration in the biological activity. Int Immunopharmacol. May 2023;118:110113. doi:10.1016/j.intimp.2023.110113. PMID: 37028279. https://pubmed.ncbi.nlm.nih.gov/37028279/
- Tao N, Xu X, Ying Y, Hu S, Sun Q, Lv G, Gao J. Thymosin alpha1 and Its Role in Viral Infectious Diseases: The Mechanism and Clinical Application. Molecules. Apr 17 2023;28(8)doi:10.3390/molecules28083539. PMID: 37110771; PMCID: PMC10144173. https://pubmed.ncbi.nlm.nih.gov/37110771/
- Guo Y, Chang H, Li J, Xu XY, Shen L, Yu ZB, Liu WC. Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/Akt/mTOR signaling pathway. Apoptosis. Aug 2015;20(8):1109-21. doi:10.1007/s10495-015-1138-9. PMID: 26002438. https://pubmed.ncbi.nlm.nih.gov/26002438/
- Cao M, Wang G, Xie J. Immune dysregulation in sepsis: experiences, lessons and perspectives. Cell Death Discov. Dec 19 2023;9(1):465. doi:10.1038/s41420-023-01766-7. PMID: 38114466; PMCID: PMC10730904. https://pubmed.ncbi.nlm.nih.gov/38114466/
- Guo CL, Mei JD, Jia YL, Gan FY, Tang YD, Liu CW, Zeng Z, Yang ZY, Deng SY, Sun X, Liu LX. Impact of thymosin alpha1 as an immunomodulatory therapy on long-term survival of non-small cell lung cancer patients after R0 resection: a propensity score-matched analysis. Chin Med J (Engl). Nov 3 2021;134(22):2700-2709. doi:10.1097/CM9.0000000000001819. PMID: 34732663; PMCID: PMC8631386. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8631386/