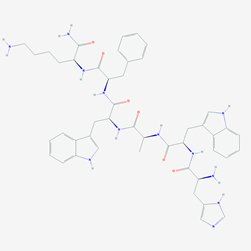
Overview of Ipamorelin and Peptide Research
Peptide Sequence: Aib-His-D-2Nal-D-Phe-Lys
Molecular Formula: C38H49N9O5
Molecular Weight: 711.868 g/mol
Ipamorelin Peptide Mode of Action
The GHS receptor, also called the ghrelin receptor, is highly expressed in the anterior pituitary gland and hypothalamus. Growth hormone is released from the anterior pituitary gland as the agonists bind to the GHS-R in the pituitary gland. Five of the most active GHS-R binding amino acids appear to be present in Ipamorelin, making this short peptide the smallest known GHS-R agonist. The exposure to Ipamorelin in animal models reportedly resulted in the stimulation and the release of neuropeptide Y, which may lead to an increased appetite, decreased pain perception, and changes in energy metabolism.
Food Intake
The specific receptors required for stimulating the release of neuropeptide Y are situated in the hypothalamus, and the binding to the GHS-R might trigger the release of this peptide. The release of neuropeptide Y is associated with an increase in food intake. Therefore, it might alter food preferences, thereby increasing the storage of energy as fat.
Pain Perception
Research suggests the potential of Ipamorelin in reducing neuropathic pain, the pain associated with nerve dysfunction, and reducing visceral pain, the gastrointestinal tract, and organ pain. Research studies in rats indicate a reduction in pain perception by as much as 2-fold.
Bone
Some research studies in rats suggest an association of Ipamorelin with the regulation of bone turnover and promotion of mineralization of existing bones and the formation of new bones. Research in rats suggests the effectiveness of Ipamorelin in offsetting the effects of substances like corticosteroids and certain conditions considered to compromise bone density and increase bone formation by as much as four-fold. In addition, Ipamorelin has been suggested to prevent aberrant fat deposition seen with high corticosteroid levels and prevent bone loss.
Ipamorelin Peptide & Heart Function
The relationship between ghrelin and heart function is a complicated one. However, research in cell cultures and research in animal models indicate that ghrelin may help protect the heart after a heart attack or other cardiac damages. Furthermore, it might also be that ghrelin produced in minute amounts in the vascular system may help in reducing the scar formation and the successive hypertrophy of the heart. This might thereby put a stop to the long-term consequences of heart damage and also heart failure.
Gender Differences in Ipamorelin Peptide Responses
Research studies conducted in mice, rats, and pigs suggest that the peptide may exhibit objectively larger benefits in females than males. The relative lack of testosterone in females compared to males has been hypothesized to be the foundation for why the peptide may potentially be more effective in females. The lower levels of testosterone have been associated with a decrease in muscle anabolism and lower bone mineral density. Research studies are ongoing to confirm any other reasons associated with the higher potential effectiveness of the peptide in females.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.