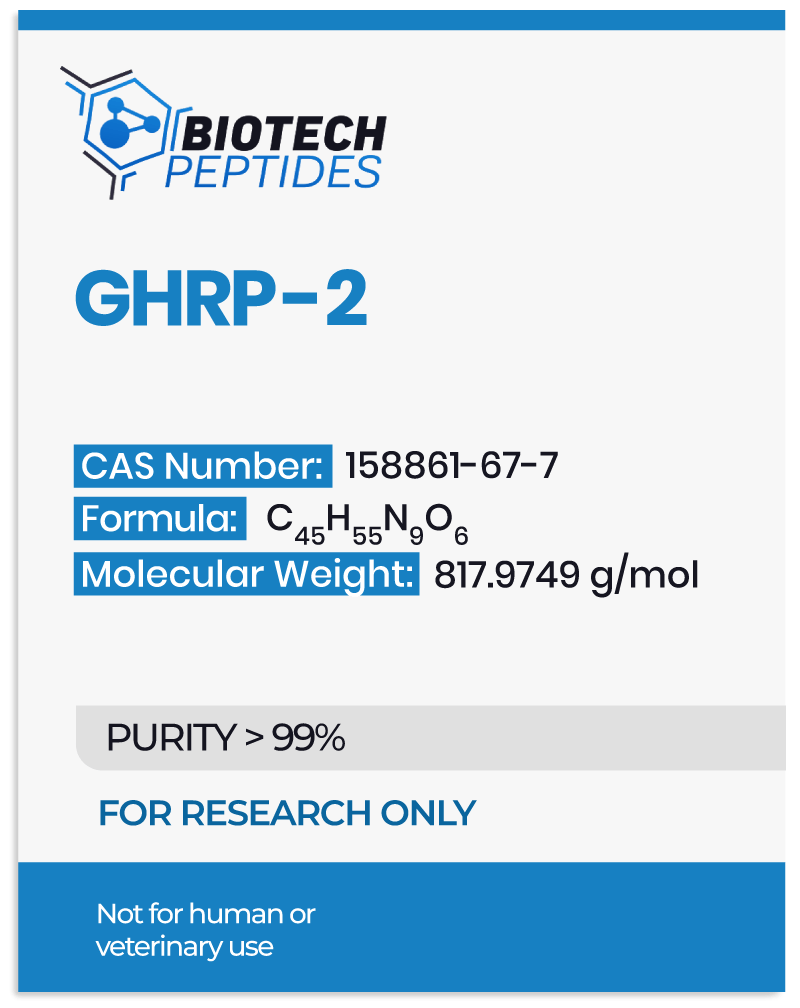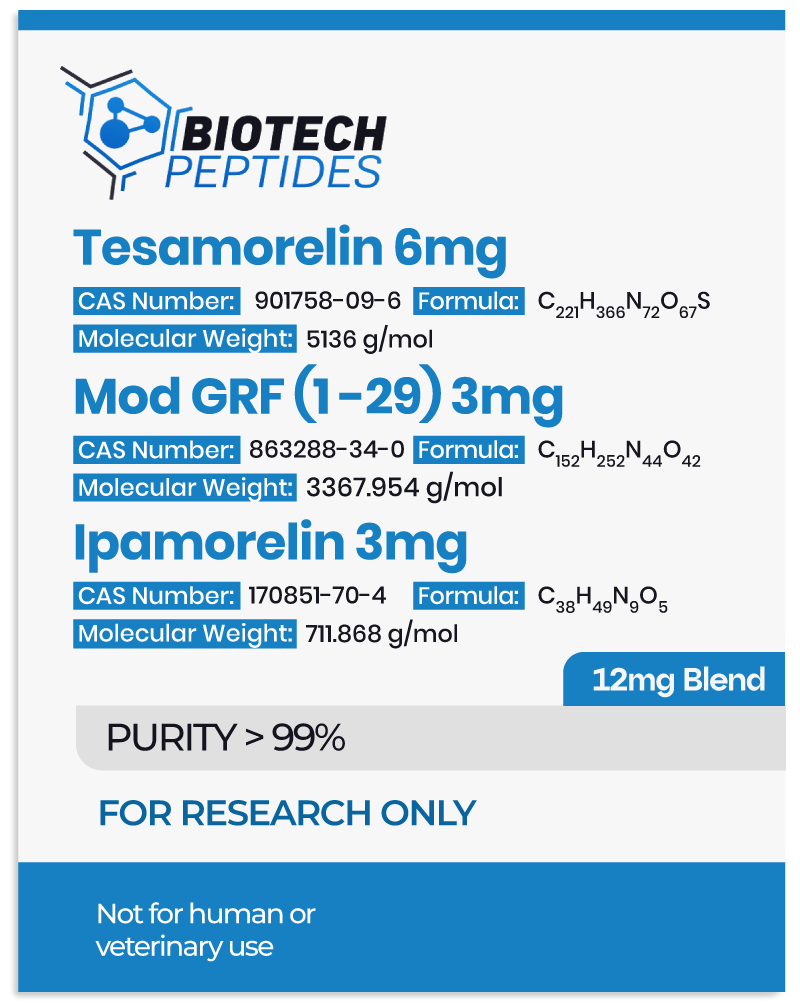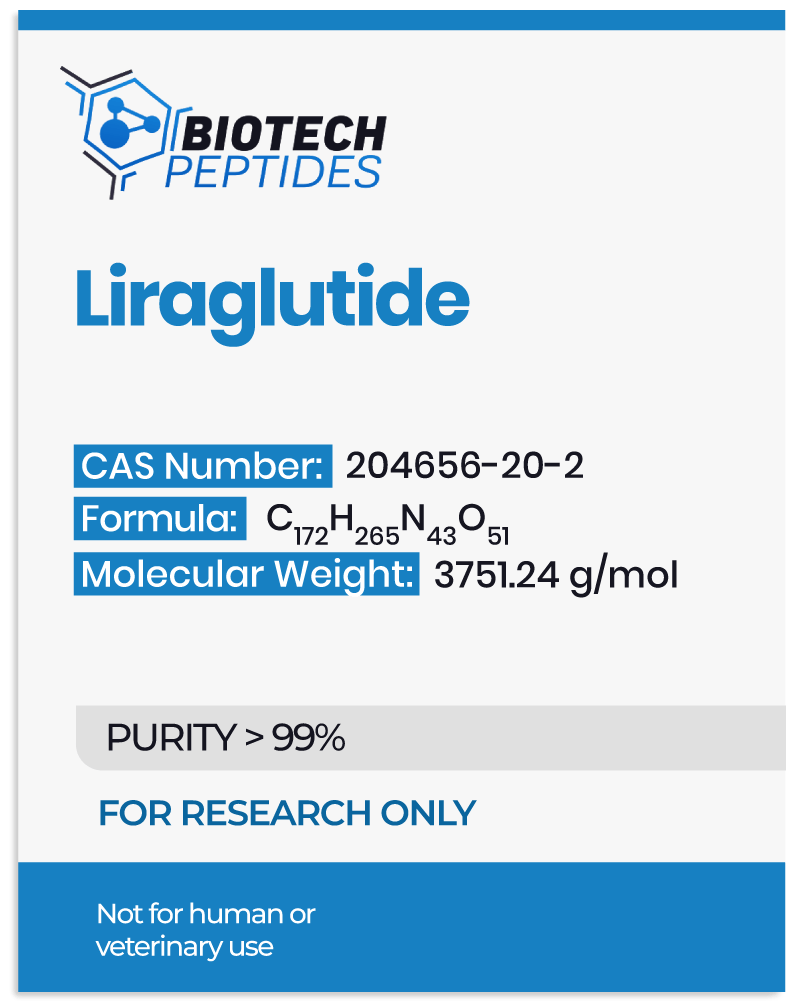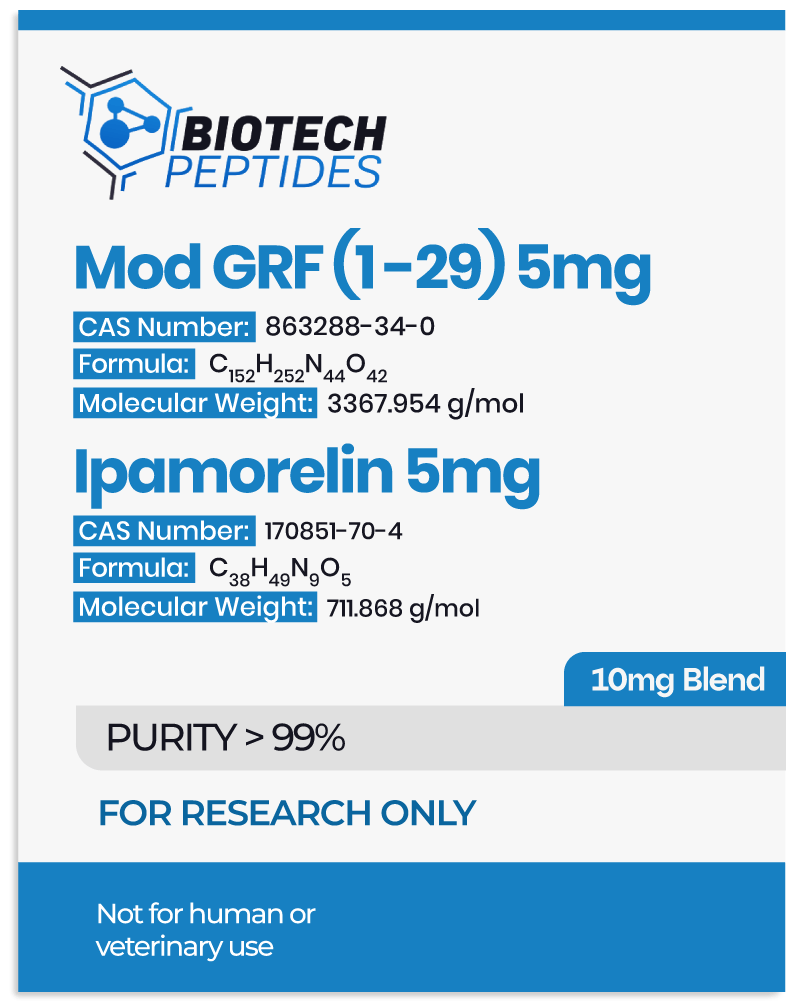
Pralmorelin Peptide and Growth Hormone Secretegogue Receptors (GHS-Rs)
Studies suggest that Pralmorelin primarily exerts its impacts through its interaction with GHS-Rs, which are widely expressed in the hypothalamus and pituitary gland. Upon binding, it is believed to induce a conformational change in the receptor, activating intracellular signaling cascades mediated by G-proteins. This process may initiate the phospholipase C (PLC) pathway, leading to the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol triphosphate (IP3) and diacylglycerol (DAG). The proposed subsequent release of calcium ions and activation of protein kinase C (PKC) may facilitate GH secretion from somatotroph cells in the anterior pituitary.[2, 3]
Additionally, Pralmorelin appears to stimulate the cyclic adenosine monophosphate (cAMP) pathway, further amplifying GH synthesis and release. In bovine studies, interactions with calcium channels and growth hormone release factor receptors have also been suggested as contributing factors. Apart from GH regulation, Pralmorelin is speculated to influence neuropeptide signaling, particularly by supporting the expression of hunger hormone-stimulating peptides like neuropeptide Y (NPY) and agouti-related peptide (AgRP) while concurrently suppressing melanocyte-stimulating hormone (α-MSH). These mechanisms collectively implicate Pralmorelin in metabolic and neuroendocrine functions beyond GH secretion.
Contents:
- Scientific and Research Studies
- Pralmorelin Peptide and Growth Hormone Deficiency Diagnosis
- Combined Studies with TRH and GnRH
- Pralmorelin Peptide and Hunger Hormone Signal Modulation
- General Pharmacological Impacts
- Pralmorelin Peptide and Antioxidative Properties
- Pralmorelin Peptide and Muscular Tissue Preservation
- Pralmorelin Peptide and Inflammation
- References
Scientific Research and Studies
Pralmorelin Peptide and Growth Hormone Deficiency Diagnosis
The insulin tolerance test (ITT) is employed to assess growth hormone (GH) deficiency; however, this method is associated with potentially severe adverse impacts and ancillary impacts. To address these limitations, a clinical study was carried out to determine the possible diagnostic potential of Pralmorelin as an alternative method for evaluating GH deficiency.
As per the study reports[4], a cohort of 135 research models initially underwent ITT, identifying 77 research models with normal GH responses and 58 with peak GH levels below 3 ng/mL. Following an overnight fasting period, the cohort was introduced to Pralmorelin, with blood samples collected at regular intervals. Researchers state that a distinct peak in GH levels was observed one-hour post peptide introduction in all subjects, with no significant differences based on sex.
That said, a marginal reduction in GH response was noted in research models with higher body mass index (BMI) and advanced cellular age. These findings were reproducible upon repeated evaluation, suggesting consistently elevated GH levels in functional research models compared to those with confirmed GH deficiency. The study findings suggest that Pralmorelin may serve as a reliable diagnostic tool for severe GH deficiency. Its efficacy varies slightly with adiposity and cellular age.
In a separate study[5], the diagnostic performance of Pralmorelin was compared to conventional stimulatory agents such as arginine and L-dopa in GH deficiency (GHD). Twenty-four research models of GHD and previously introduced to at least one conventional agent were enrolled in the study, which evaluated the introduction of Growth Hormone-Releasing Hormone (GHRH) and GHRP-2. The introduction of Pralmorelin markedly elevated serum GH levels. Among the 21 research models that exhibited a strong GH response, GHRH and GHRP-2 were simultaneously introduced. Subsequently, 15 of these research models underwent intranasal introduction of GHRP-2, which similarly induced a significant GH response.
All research models exposed to Pralmorelin in this study indicated good tolerance, suggesting a favorable profile in younger research models. Notably, reports suggest that Pralmorelin appears to exhibit a distinct advantage as a predictor of pituitary GH secretory capacity, a characteristic not observed with conventional diagnostic agents. These findings highlight the potential of Pralmorelin as an impactful and noninvasive diagnostic tool for researching GH deficiency.
Combined Studies with TRH and GnRH
A clinical investigation[6] studied the impacts of Pralmorelin, Thyrotropin-Releasing Hormone (TRH), and Gonadotropin-Releasing Hormone (GnRH), both individually and in combination, in subjects with prolonged hyposomatotropism, hypogonadism, or hypothyroid complications. The study recruited 33 male research models to assess their endocrine responses to different hormonal stimulation regimens. Over five days, subjects were assigned to one of four groups: placebo, the hourly introduction of Pralmorelin, the combined introduction of Pralmorelin and TRH every hour, or a combination of Pralmorelin, TRH, and GnRH every 90 minutes.
Serum samples were collected on both the first and last nights of the study for endocrine analysis. Results suggest that the combination of GHRP-2, GnRH, and TRH elicited the most pronounced activation of the growth hormone, thyroid-stimulating hormone, and luteinizing hormone axes, potentially influencing metabolic pathways. In contrast, Pralmorelin alone produced minimal endocrine impacts, and, according to the reports, the combination of Pralmorelin and TRH induced only partial hormonal activation compared to the triple-hormone regimen. These findings suggest a potential synergistic interaction between Pralmorelin, GnRH, and TRH in supporting hormonal responses, suggesting their possible role in managing endocrine deficiencies and related metabolic disturbances.
Pralmorelin Peptide and Hunger Hormone Signal Modulation
This controlled experiment studies the potential role of Growth Hormone-Releasing Peptide-2 (GHRP-2) in hunger hormone signal regulation. Seven functional male research models were allocated into two groups, with one group receiving a continuous subcutaneous infusion of GHRP-2 and the other receiving a saline placebo over 4.5 hours. Following the infusion, all research models were provided unlimited access to nourishment, and caloric intake was measured to assess differences between the groups.
Results suggested a significant increase in caloric intake among research models exposed to Pralmorelin, with an average intake approximately 36% higher than that of the control group. This reported increase in hunger hormone signals was accompanied by a concurrent elevation in circulating growth hormone (GH) levels, based on which the researchers concluded that “GHRP-2, like ghrelin, increases food intake, suggesting that GHRP-2 [may be] a valuable tool for investigating ghrelin effects on eating behavior.”
These findings support the hypothesis that Pralmorelin may impact hunger hormone stimulation, potentially through its interaction with ghrelin receptors and downstream neuroendocrine signaling pathways.
General Pharmacological Impacts
Preclinical investigations[8] have studied the pharmacological profile of Pralomorelin in animal models. Studies conducted in rabbits and guinea pigs suggest that Pralmorelin does not significantly impact central nervous system function. However, a mild increase in motility was observed in the isolated rabbit ileum, and concentration-dependent support of contraction was noted in the isolated guinea pig ileum.
Beyond these gastrointestinal impacts, no substantial alterations were reported in respiratory, digestive, renal, or circulatory system functions. Researchers concluded that the peptide “has no serious general pharmacological effects at [concentration] levels showing GH-releasing activity in the experimental animals,” and the peptide is speculated to support the diagnosis of “serious GH deficiency and short stature.”[8]
Pralmorelin Peptide and Antioxidative Properties
Studies suggest that Pralmorelin may exert antioxidative impacts, with data suggesting its interaction with CD36, a receptor involved in the uptake of oxidized low-density lipoprotein (OxLDL). This interaction might potentially limit the cellular absorption of OxLDL, a well-regarded contributor to atherogenesis. In murine models deficient in the ApoE gene (ApoE⁻/⁻), prolonged Pralmorelin exposure over 12 weeks was observed to increase circulating insulin-like growth factor-I (IGF-I) levels by approximately 1.2 to 1.6 times baseline values. Additionally, a 66% reduction in circulating interferon-gamma levels was reported.
While Pralmorelin did not appear to significantly alter the extent of atherosclerotic plaque formation, it appeared to reduce superoxide production within the aorta, as supported by data collected concerning dihydroethidium staining. Furthermore, Pralmorelin exposure resulted in a 92% reduction in aortic gene expression of 12/15-lipoxygenase, as well as a downregulation of interferon-gamma and macrophage migration inhibitory factor. Observations in cultured aortic smooth muscle cells suggest that Pralmorelin may counteract peroxide production induced by OxLDL, mitigate IGF-I receptor suppression, and potentially inhibit apoptosis. In macrophages exposed to OxLDL, the peptide is hypothesized to reduce lipid accumulation, further supporting its antioxidative and protective potential against proatherogenic agents.[9]
Pralmorelin Peptide and Muscular Tissue Preservation
Murine models of thermal injury suggest that Pralmorelin may play a role in mitigating muscular tissue catabolism by reducing proinflammatory markers such as interleukin-6 (IL-6) and E3 ubiquitin ligases (MuRF-1 and MAFbx), both of which are implicated in muscular tissue degradation under critical conditions. Additionally, researchers propose that Pralmorelin may directly attenuate total muscle protein breakdown, indicating a potential muscle-sparing impact. Case studies further suggest that the peptide may contribute to muscular tissue hypertrophy and mass gain.[11]
Pralmorelin Peptide and Inflammation
To further investigate Pralmorelin’s impacts on oxidative stress and inflammation, murine models of acute lung injury were studied following peptide introduction. Findings suggest that Pralmorelin exposure may reduce pulmonary edema, neutrophil infiltration, and proinflammatory cytokine levels. Additionally, the peptide appears to suppress nuclear factor-kappa B (NF-κB) activation, a key regulator of inflammatory cascades often associated with tissue damage. These findings[12] suggest that Pralmorelin may exert protective impacts against inflammation-induced tissue injury.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.
References:
- Garcia JM, Merriam GR, Kargi AY. Growth Hormone in Aging. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA): MDText.com https://www.ncbi.nlm.nih.gov/books/NBK279163/
- Yin, Y., Li, Y., & Zhang, W. (2014). The growth hormone secretagogue receptor: its intracellular signaling and regulation. International journal of molecular sciences, 15(3), 4837–4855. https://doi.org/10.3390/ijms15034837
- Sinha, D. K., Balasubramanian, A., Tatem, A. J., Rivera-Mirabal, J., Yu, J., Kovac, J., Pastuszak, A. W., & Lipshultz, L. I. (2020). Beyond the androgen receptor: the role of growth hormone secretagogues in the modern management of body composition in hypogonadal males. Translational andrology and urology, 9(Suppl 2), S149–S159. https://doi.org/10.21037/tau.2019.11.30
- Roh SG, He ML, Matsunaga N, Hidaka S, Hidari H. Mechanisms of action of growth hormone-releasing peptide-2 in bovine pituitary cells. J Anim Sci. 1997 Oct;75(10):2744-8. doi: 10.2527/1997.75102744x. PMID: 9331879. https://pubmed.ncbi.nlm.nih.gov/9331879/
- Asad Rahim, Stephen M. Shalet, in Growth Hormone Secretagogues, 1999. Does desensitization to growth hormone secretagogues occur? https://www.sciencedirect.com/
- Van den Berghe G, Baxter RC, Weekers F, Wouters P, Bowers CY, Iranmanesh A, Veldhuis JD, Bouillon R. The combined administration of GH-releasing peptide-2 (GHRP-2), TRH and GnRH to men with prolonged critical illness evokes superior endocrine and metabolic effects compared to treatment with GHRP-2 alone. Clin Endocrinol (Oxf). 2002 May;56(5):655-69. doi: 10.1046/j.1365-2265.2002.01255.x. PMID: 12030918. https://pubmed.ncbi.nlm.nih.gov/12030918/
- Laferrère, Blandine et al. Growth hormone-releasing peptide-2 (GHRP-2), like ghrelin, increases food intake in healthy men. The Journal of Clinical Endocrinology and Metabolism vol. 90,2 (2005): 611-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2824650/
- Furuta S, Shimada O, Doi N, Ukai K, Nakagawa T, Watanabe J, Imaizumi M. General pharmacology of KP-102 (GHRP-2), a potent growth hormone-releasing peptide. Arzneimittelforschung. 2004;54(12):868-80. doi: 10.1055/s-0031-1297042. PMID: 15646371. https://pubmed.ncbi.nlm.nih.gov/15646371/
- Titterington JS, Sukhanov S, Higashi Y, Vaughn C, Bowers C, Delafontaine P. Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in ApoE-/- mice but does not reduce atherosclerosis. Endocrinology. 2009 Dec;150(12):5478-87. doi: 10.1210/en.2009-0283. Epub 2009 Oct 9. PMID: 19819949; PMCID: PMC2795722. https://pmc.ncbi.nlm.nih.gov/articles/PMC2795722/
- Sheriff, S., Joshi, R., Friend, L. A., James, J. H., & Balasubramaniam, A. (2009). Ghrelin receptor agonist, GHRP-2, attenuates burn injury-induced MuRF-1 and MAFbx expression and muscle proteolysis in rats. Peptides, 30(10), 1909–1913. https://doi.org/10.1016/j.peptides.2009.06.029
- Sigalos, J. T., & Pastuszak, A. W. (2018). The Safety and Efficacy of Growth Hormone Secretagogues. Sexual medicine reviews, 6(1), 45–53. https://doi.org/10.1016/j.sxmr.2017.02.004
- Li, G., Li, J., Zhou, Q., Song, X., Liang, H., & Huang, L. (2010). Growth hormone-releasing peptide-2, a ghrelin agonist, attenuates lipopolysaccharide-induced acute lung injury in rats. The Tohoku journal of experimental medicine, 222(1), 7–13. https://doi.org/10.1620/tjem.222.7