Triptorelin and Research in Prostate Cancer
Researchers suggest that Triptorelin may be of most interest in studies on prostate cancer, where exposure to the peptide has been speculated to lower cancer growth by inducing a dip in endogenous testosterone levels. Triptorelin appears to decrease the mortality rate in hormone-sensitive prostate cancer to less than 5% in experimental versus control group comparisons.
Recent research posits that GnRH in symbiont with radiotherapy may potentially exhibit similar action to a total androgen blockade. The blockade of androgen may induce ancillary downstream action, which may potentially be circumvented.
Triptorelin exhibited apparently positive results in reducing urinary tract symptoms in research models of prostate cancer, with the research team reporting that the peptide may have contributed to reducing the frequency of the symptoms from about 54% to about 12%.
Scientific Studies
Triptorelin and Reproduction, Fertility
Research also suggests that Triptorelin could potentially reduce the prevalence of early menopause in research models that have undergone chemotherapy by approximately 17%. The exposure of this peptide in research models with Adenomyosis might increase the degree of spontaneous pregnancy and potentially improve the outcomes of the disease itself. Identical benefits may be evident in research models with endometriosis.
In endometriosis, triptorelin might act to reduce pain by potentially causing a decrease in the number of nodules in the disease. Research speculates that Triptorelin could potentially improve the outcomes of laparoscopic surgery for endometriosis. It is believed to be specifically effective in potentially increasing the rates of pregnancy after surgery.
In In research models with colorectal endometriosis, Triptorelin appears to potentially decrease pain in about 80% of the subjects and diarrhea in about 60%.
Triptorelin and Immune Function
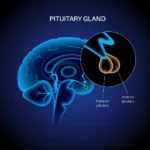
Research in mouse models suggests that LHRH might potentially impact the thymus and the immune system. Cell aging in research models may reduce the LHRH binding sites on the thymus, potentially resulting in a 50% reduction in thymic mass and function.
The LHRH agonist, Triptorelin, may potentially improve proliferation within the thymus and may induce a turnaround in cell aging impacts to some extent.
Disclaimer: The products mentioned are not intended for human or animal consumption. Research chemicals are intended solely for laboratory experimentation and/or in-vitro testing. Bodily introduction of any sort is strictly prohibited by law. All purchases are limited to licensed researchers and/or qualified professionals. All information shared in this article is for educational purposes only.